

Three gases that are distinctive in their appearance, and do not closely resemble anything else:
  |
  |
  |
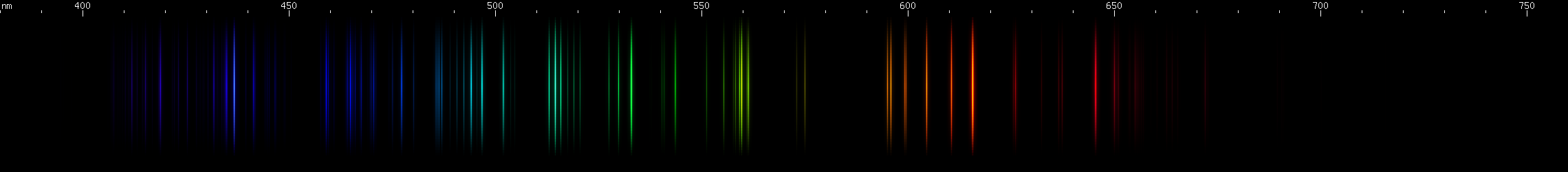
Oxygen: Can be recognized by its two green brightnesses, one of them rather a kelly green (~530nm) and the other more of a chartreuse (~560nm), plus the orange-red group of lines (~595-615nm) and deeper red line (~645nm), plus a lot of brightness in the blue-violet. Oxygen does not emit any intense visible lines, and the pictured spectrum was difficult to photograph, but oxygen does produce two strong near infrared lines, near 777nm and 845nm. Each of these is actually a very close triplet. Oxygen normally occurs as O2 which, being a molecule, would not have the same spectrum as atomic O. Fortunately, the bond dissociation of O2 is small enough that oxygen in a spectrum tube emits only atomic O lines, with little or no light from molecular bands.
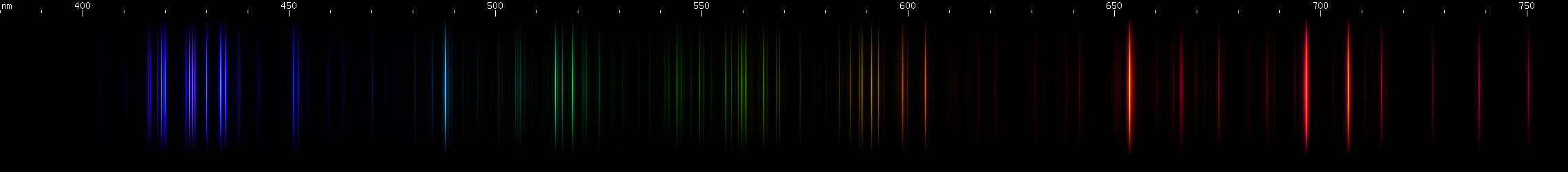
Argon: It's hard to mistake the spread-out pattern of bright lines from orange to almost infrared. Add the violet grouping and the blue-green line, and argon is an easy to recognize spectrum. But argon's brightest lines are in the infrared and the vacuum ultraviolet, and its visible lines tend to get drowned out in mixtures. So while argon is the gas used in most mercury lamps, you won't see any argon lines unless you have an infrared camera and even then many lamps still won't emit easily observable argon infrared. Argon is, however, a moderately strong UV-A emitter, and has been used in glow lamps to produce a low intensity UV emission in blacklight wavelengths.
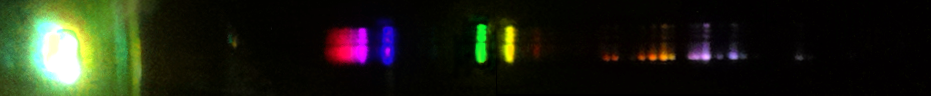
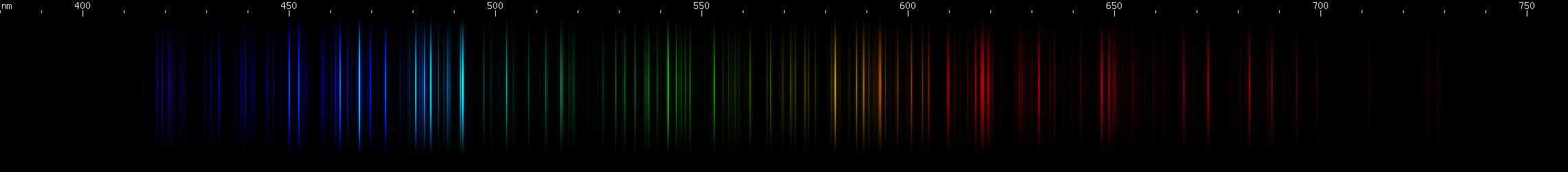
Xenon: The pattern of blue lines is distinctive here, along with the high variability of brightness of its green and orange lines. The green and orange lines indicate ionization, so depending how much energy each xenon atom is absorbing on average, we can see a strong effect in the brightnesses of such lines relative to the strong blue lines, which are from neutral xenon. In the photo, you can see at the top, near the electrode, there is proportionally much more green than underneath in the narrow part of the tube that's designed for observation. Note that the spectrum of a xenon strobe lamp is not the same as that of a xenon spectrum tube, pictured. Specifically, the strobe lamp spectrum contains an intense white continuum of wavelengths, presumably a blackbody effect from electricity heating the gas. You may be able to resolve hundreds or even thousands of lines superimposed over this continuum, but nothing in a recognizable pattern. The best way to recognize a xenon gas discharge is from the blue lines, while the best way to recognize a xenon strobe is by the extremely short duration pulses of continuous spectrum white light.
Next: Alkali Metals