
Series of neutral sodium (Na I).
Recall that for hydrogen, the visible lines are all part of a series, with a bright alpha line followed by beta, gamma, etc lines progressively dimmer and at progressively shorter wavelengths, getting closer together towards a series limit. Recall also that sodium's spectrum consists of series: one where the amber 590nm doublet is alpha, the remainder of the lines in the ultraviolet; and two series with their alphas in the infrared and pairs of visible lines converging towards the violet region.

To help us understand alkali metal spectra, let's label sodium's various series. The amber and ultraviolet lines belong to the P series, named for the 2P term of the upper energy level of each line. In the case of alkali metals, we can consder the letter of the term (S, P, D, F) as mostly synonymous with the orbital of the outer electron (s, p, d, f); the superscript 2 means doublet. Sodium's S series begins in the infrared and has the orange-red line (a doublet) as its beta; the dim emerald-green line as gamma, and the rest of its lines are the weaker member of each pair converging. Sodium's D series also has its alpha in the infrared, but its beta is the greenish-yellow line (a compound doublet), and its gamma through infinity are the stronger members of the converging pairs. Sodium also has an F series based entirely in the infrared. So using this terminology, when talking about neutral lines of alkali metal spectra, we can just say something like "D-alpha" or "P-beta" and it's clear which line of which series is meant.
(A compound doublet is three lines. Technical explanation, which you are free to skip over: Since all of the energy level series are doublets, all of the levels will be split in two by the electron's spin, except for S terms which are always singular. An electron's spin is one half, and it sums with one of the electron's other quantum numbers so that you get terms like 2P1/2 and 2P3/2; 2D3/2 and 2D5/2, etc. The rule is 3/2 can transition to 1/2 or to 3/2, and 5/2 can transition to 3/2, but 5/2 cannot transition to 1/2. So you get 3 lines from a compound doublet. Higher multiplets exist, which we see in elements with complex spectra. Not to worry, I won't spring a compound octuplet on you, but it can have up to 21 lines!)
Besides sodium, there are four other stable alkali metals. Not showing francium as only 3 visible lines are known. In fact, I only have photos for two of these and they, well, leave a lot to be desired, so I'm just going to show you the computer generated line spectrum views.
 |
 |
 |
 |
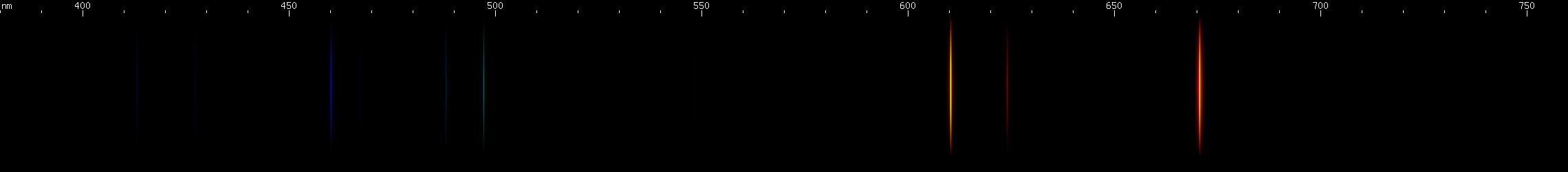
Lithium: The strong cherry-red line at 670.8nm is the P-alpha line, analogous to sodium's bright amber doublet; the rest of the series is in the UV. The D-alpha is visible at 610.4nm, but the S-alpha is in the infrared; the D-beta and S-beta are visible as blue (460nm) and aqua (497nm) lines, respectively. The S and D gamma lines are faintly visible in the violet region. The remaining blue and blue-green lines are mostly from Li II. There is also a weak line around 624nm; this is a "forbidden" line, i.e. one that violates one of the rules of which transitions are allowed. It occurs from a transition from one 2P energy level to another; normally P can only transition to S or D, since the rule is the electron or the term moves one space over in either direction. Although "forbidden" lines are generally much weaker than "allowed" lines from the same upper energy levels, "forbidden" lines do occur.
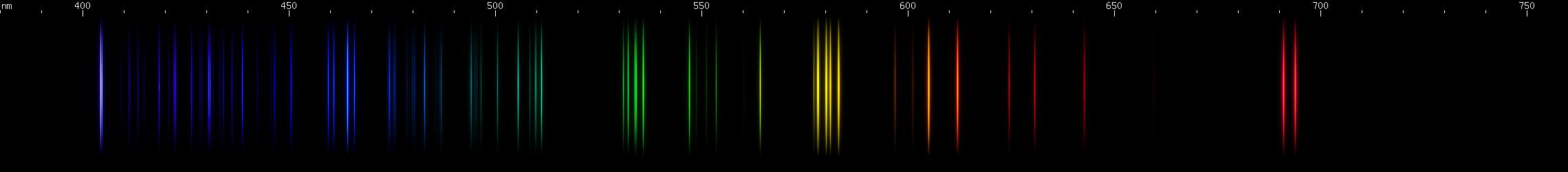
Potassium: We see a trend begin where the lines of the series move towards longer wavelengths as we go down the periodic table. Potassium's P-alpha doublet is located at 766.5nm and 769.9nm compared to sodium's 590nm, and this longer wavelength can be visually observed as a pair of very hard to see dull red lines. The P-beta is a 404nm deep violet doublet. This is the strongest component of potassium's violet color in a flame test. The D and S alphas are located in the infrared, but we can see their betas clustered together near 690nm, gammas clustered together near 580nm, and further groups of lines near 535nm, 510nm, and 495nm. There is a forbidden line at 464nm from a transition from 2D to 2S. A scattering of K II lines may be visible, especially in the violet region.
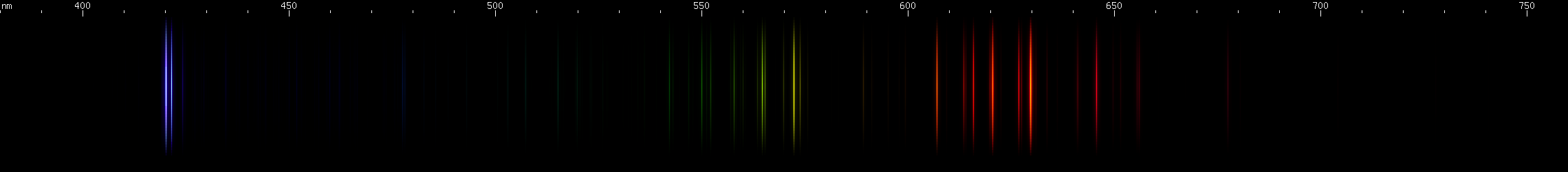
Rubidium: The P-alpha doublet occurs at 780nm and 795nm, which is possible to see but difficult. The beta doublet is in the violet near 420nm. The D and S alphas occur in the infrared, with their betas in the far-red near 762-776nm (D) and 728-741nm (S). The remaining lines of these series converge towards a limit in the blue-green region, but the pattern is hard to see because some red and green Rb II lines intermingle.
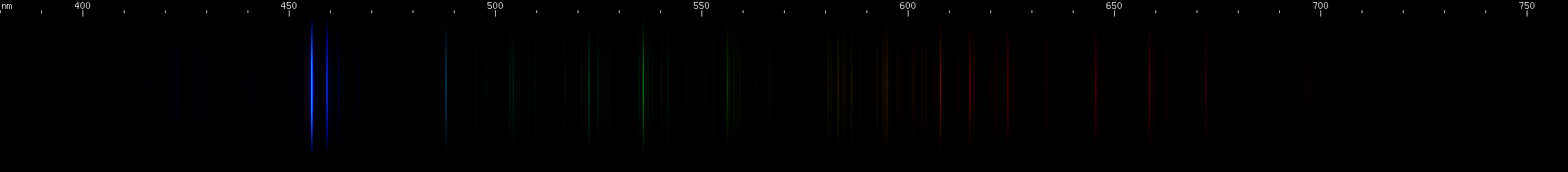
Cesium: The P-alpha doublet is solidly in the infrared, but its beta occurs in the blue region at 455nm and 459nm. The alpha and beta lines of the D and S series are all infrared, but their remaining lines converge towards green. Scattered Cs II lines in the yellow, green, and blue-green again break up the pattern making it hard to see. Altogether the visible spectrum of cesium is dominated by that blue doublet, as is the violet color of its flame test.
Next: Recognizable Common Metals