
.png)
.png)
|Y2O3:Tb(III).png)
|Y2O3:Tb(III).png)
Now we'll look at another very common spectrum, and two of its combinations.
  |
.png) .png) |
|Y2O3:Tb(III).png) |Y2O3:Tb(III).png) |
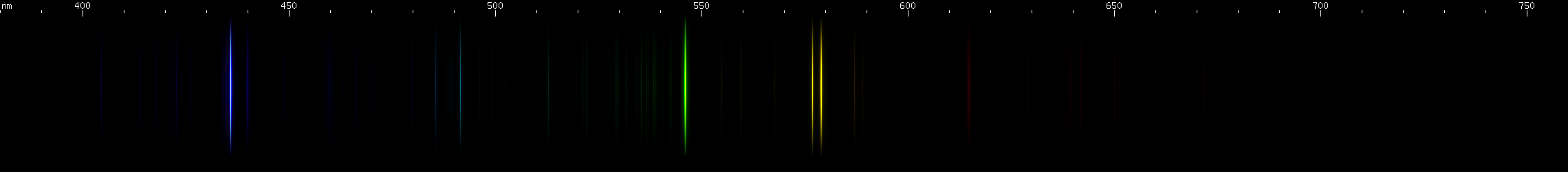
Mercury: The photo depicts a streetlamp, the old style blue-green type, which emits only mercury lines. You can tell this type of lamp because red items look khaki or black under them. All fluorescent lamps use mercury vapor as the UV source. All "neon" signs that are not some shade of red or orange, are actually colorful little fluorescent lamps using custom phosphors to make a saturated hue instead of almost white. All metal halide lamps contain mercury, as do most sodium vapor lamps. In such light sources, you will always see the pattern of yellow, green, and indigo-violet lines (the deep violet line is hard to see). When you observe such a lamp in person, memorize the exact colors of these three lines, as well as their locations relative to each other.
Mercury with Red Phosphor: To address the problem of mercury lamps giving off basically zero red light, manufacturers started adding a red phosphor, which absorbs some of the UV and fluoresces orange-red. These lamps have a sort of purplish-amber-greenish glow, a color which cannot be duplicated on a computer monitor. (Nerd moment: I swear human mesopic color perception must have some degree of rod input, since it's not like this is a saturated color beyond the monitor's CIE 1931 triangle.) The phosphor is yttrium europium orthovanadate, but it does not emit lines of yttrium, europium, or vanadium. Its spectrum is unique, arising from the interaction between the crystal lattice and the 4f electrons of the europium III ion. Such narrow bands that resemble lines are the norm in rare earth phosphors, since the crystal structure doesn't greatly perturb electrons in f orbitals. This yttrium europium orthovanadate is the very same phosphor that was most commonly used for the red component in color television CRTs. For now, just notice that the spectrum looks very much like plain mercury, but with added red and a somewhat fainter little orange brightness.
Triphosphor Fluorescent Lamp: Another type of lamp that combines mercury with a phosphor - or more specifically, three phosphors. The choice of phosphors seems to be to maximize color rendering under these lamps; the most intense emitted wavelengths are red, green, and indigo-blue, with the result that colors will be more vibrant than under other types of lighting, including daylight. The phosphor is yttrium oxide activated with europium and terbium. The terbium produces the narrow green and cyan bands, along with some of the yellow and a little bit of red. The europium produces almost all the red and most of the yellow. Notice that yttrium europium oxide has a different spectrum from yttrium europium orthovanadate, but that both are orange-red emitters. The reason for choosing orange-red instead of a deeper red is so that less electricity must be used for the same perceptual brightness.
In the 20th century, fluorescent lamps used to contain mercury vapor and a calcium halophosphate with impurities of manganese, antimony, etc that would produce a wide band of wavelengths from green to red, peaking in the yellow, and often with an additional peak or two in the blue-green and maybe blue. Around the early 1990s, compact fluorescent lamps (CFLs) became commonplace, replacing incandescent light bulbs with a small triphosphor tube. In time, triphosphor lamps had completely replaced the old halophosphate type even in those long 4-foot tubes, making halophosphate lamps hard to find. Nowadays, white LED lamps are replacing fluorescent lamps. Interestingly, the white LED spectrum has a similar overall shape to the halophosphate spectrum: narrow brightness in the indigo part of the spectrum, plus a wide swath of green, yellow, orange, and red.
 |
In all four of these mercury based lamps, you will see the familiar yellow, green, and indigo-violet lines featured prominently in their usual positions and hues.
Next: More Gases