
Semimetals are elements that do not fit cleanly into the metallic or non-metallic categories. Like metals, they may appear silvery and may alloy with metals. Like nonmetals, they usually have allotropes. They typically offer some electrical conductivity, unlike metals which are good conductors or nonmetals which are insulators.
  |
   |
  |
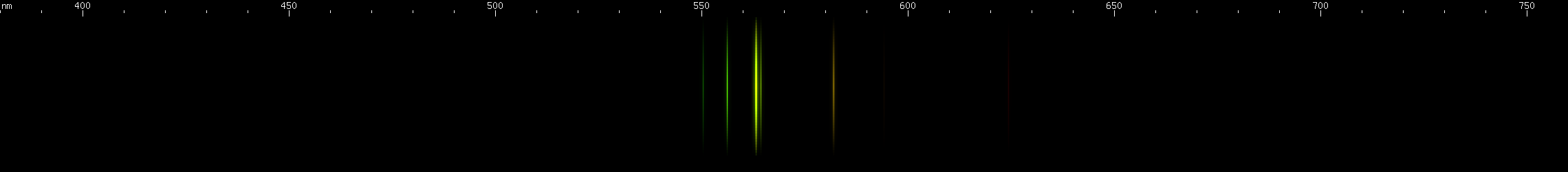
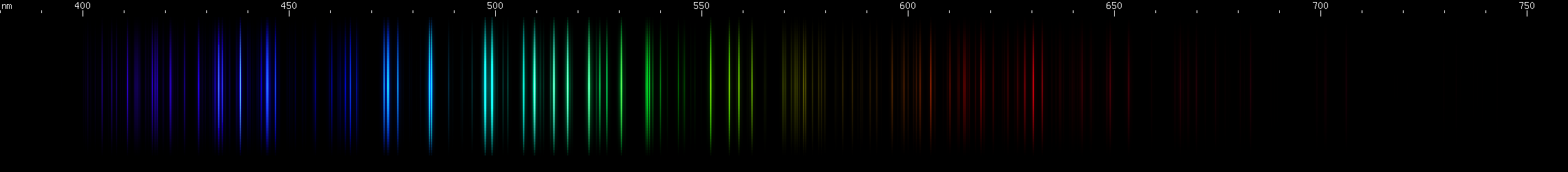
Boron: I have not been able to confirm this one visually or photographically. Boron supposedly will conduct "like a metal" if heated sufficiently, but my setup does not allow me any way to apply the necessary amount of heat. Neutral boron (B I) has no particularly intense visible lines. The strongest would be the 563nm yellow-green line. The red, orange, teal, and blue-violet lines are all from ionized B II and B III, which apparently dominate boron's visible spectrum.
Silicon: This element's spectrocopic appearance is expected to vary considerably depending on the strength of the excitation, and therefore the amount of ionization that happens. Neutral Si I produces a different pattern (above, center middle) than ionized Si II + Si III (center top). The photo depicts almost entirely Si II and Si III.
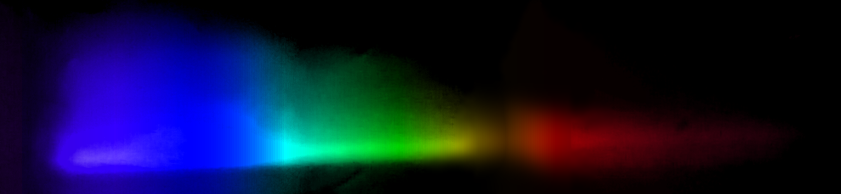
Selenium: Another element that would not conduct well enough to spark, so we used a flame. This has to be done in controlled conditions because SeO2 fumes are toxic. The photo shows where the flame touched the sample. The violet, blue, and green lines form a pattern that identifies selenium.
The next three elements have spectra that are similar in appearance. All three are toxic semimetals whose compounds often have a garlicky smell.
  |
  |
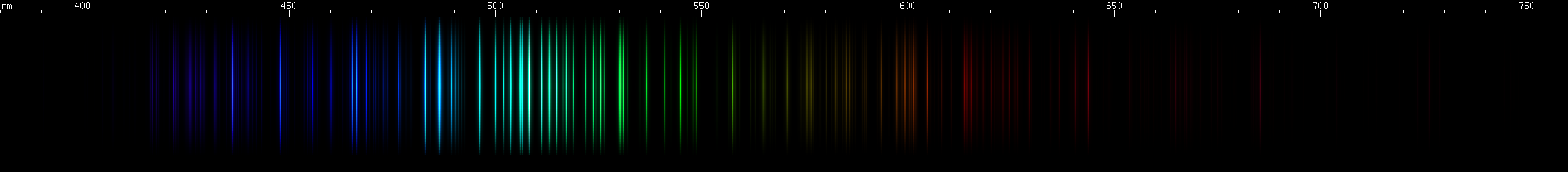  |
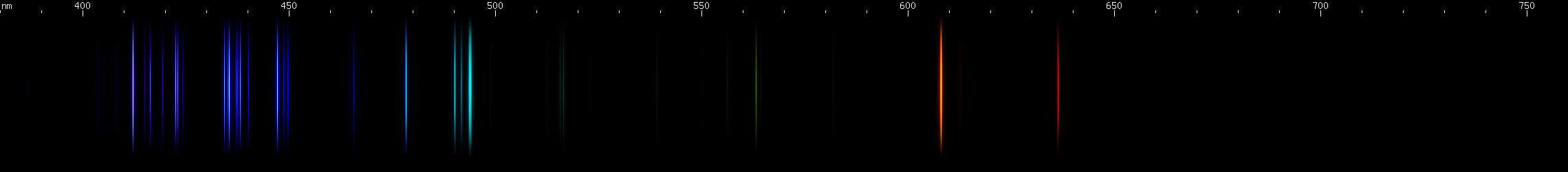
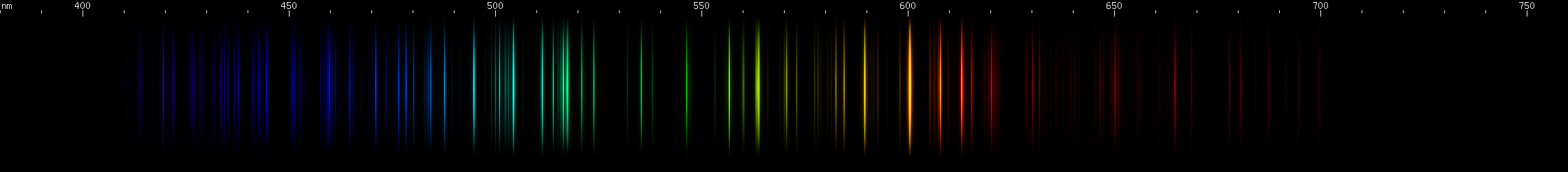
Antimony: The orange and orange-red lines of Sb II identify this one. Also look in the blue-violet for another distinctive pattern. The green lines somewhat resemble arsenic, but with stronger yellow-green.
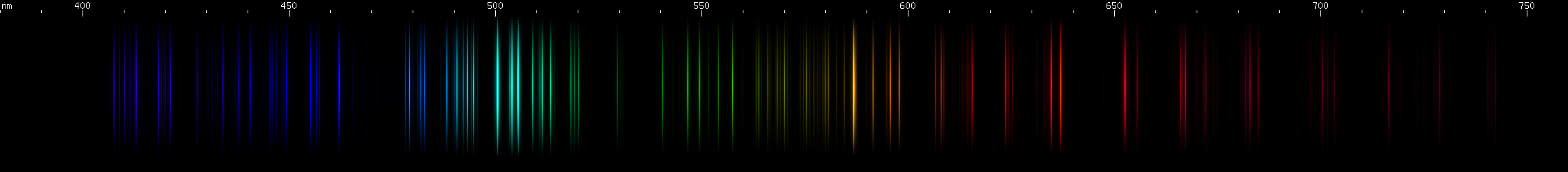
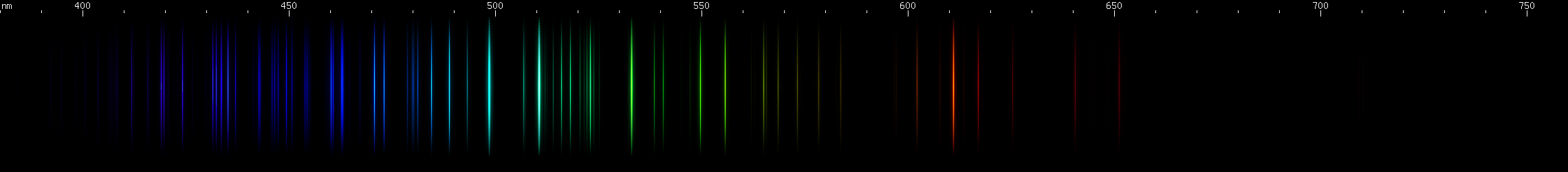
Arsenic: Dominated by teal and emerald-green lines, particularly at 511nm, with an orange-red feature less intense and less yellow than antimony's. The blue-violet region also has many moderate to bright lines, with a particular brightness near 430nm.
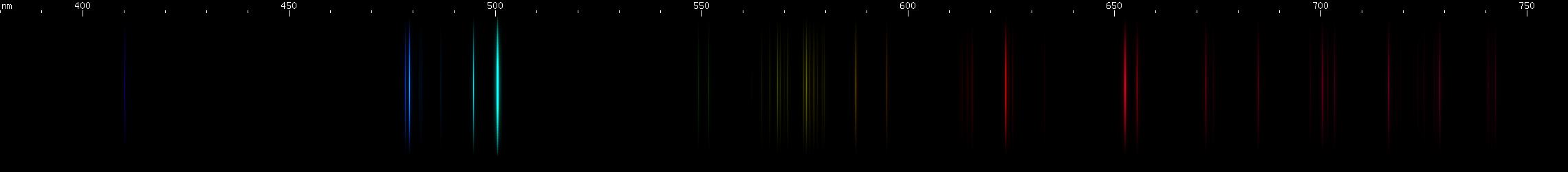
Tellurium: The teal-emerald greens are the brightest part of its visible spectrum, particularly near 508nm. A little bit of orange and red more diffuse than arsenic and much fainter than antimony. Several moderately strong blue-violet lines without any features that stand out.
Next: Lookalikes, part II