
Earlier we saw a grid of 20 spectra that all look superficially similar. All of them are characterized by many lines in the green and blue regions. Here, we will examine some of them and the relevant spectral features that visually distinguish them.
 |
  |
  |
Phosphorus: Probably emits mostly green lines with a little blue and small amounts of orange and red. I don't have a photo of phosphorus. It's dangerous, ignites in air, has to be kept wet, and if vaporized to make an arc it would react with part of the apparatus. Worst of all, it's used in illicit drug manufacture so authorities have clamped down on its availability. It's a sick world where a few unscrupulous individuals use mind altering substances to exploit the traumatized who just want to escape reality, just to make a dishonest dollar in a world where everybody must make a dollar or perish. And these knuckle headed law breakers ruin the availability of materials for amateur scientific use.
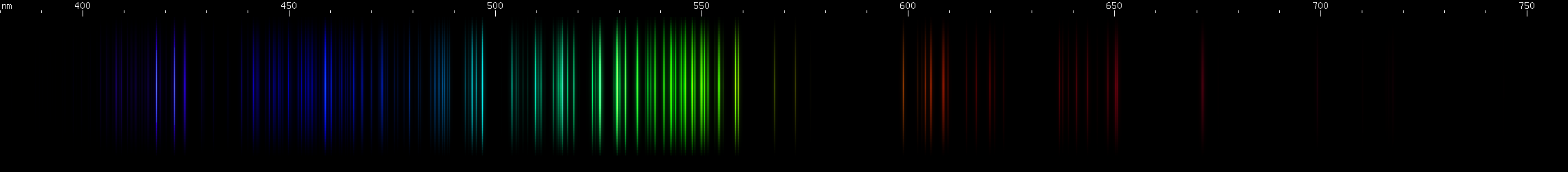
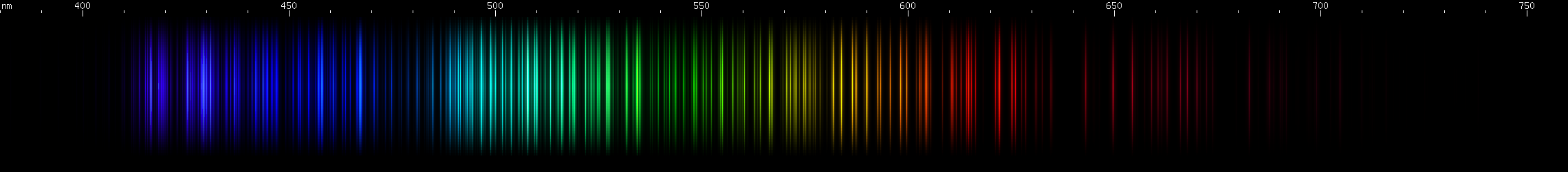
Selenium: The blue-violet lines are strongest near 410nm and 440nm. A wide swath of blue-green from 475-540nm dominates, with its brightest lines near 500nm. There's an unusual pattern of red lines near 630nm.
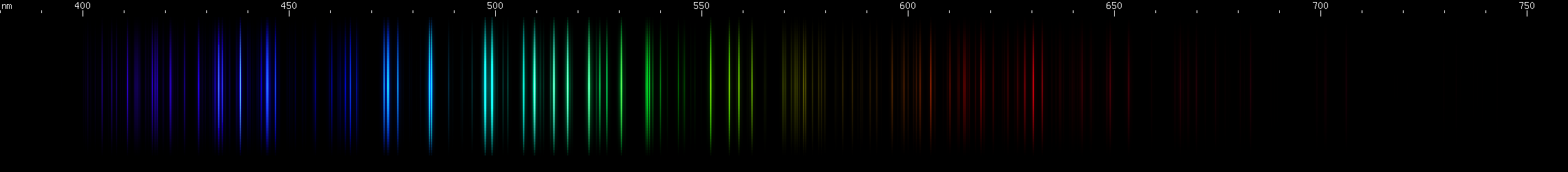
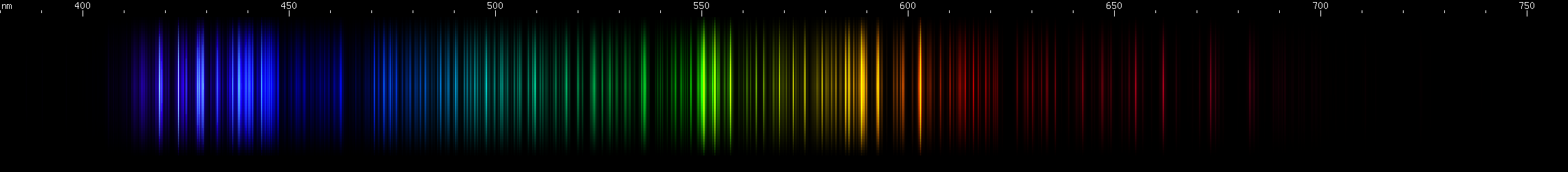
Zirconium: The green lines near 514nm and red near 612nm distinguish zirconium from similar spectra. Two indigo bright spots can also be seen on either side of 440nm.
  |
  |
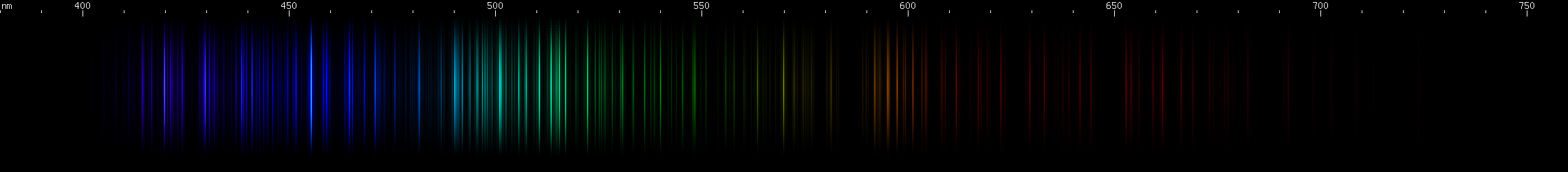  |
Niobium: Greens form a stripe between about 490-535nm. A pattern of chartreuse, yellow, orange is visible with the latter two a little less than half the distance apart vs. the former two. In the blue region are three bright lines that almost resemble the zinc triplet, except not so azure, tending more toward indigo, and with weaker lines between them. Violet lines appear in two pairs of groups.
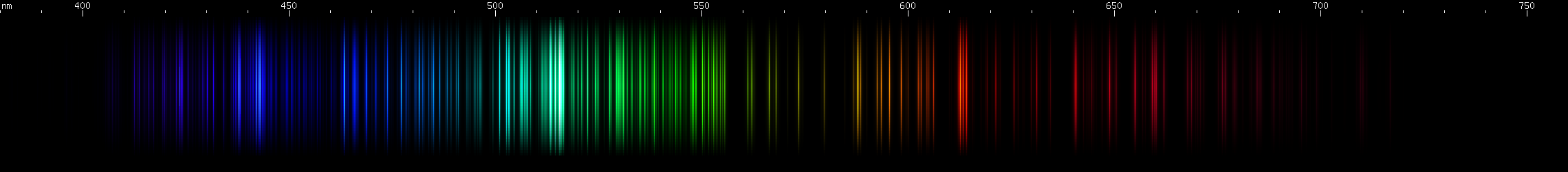
Molybdenum: A pattern of bright yellow-green, amber, and orange, with the yellow-green considerably further and brighter vs. the others. Blue-violet lines show four increasingly close together and brighter zones, with weaker intermediates, then four distinct violet lines, the second one brightest.
Ruthenium: The characteristic visible spectral feature of ruthenium is a bright blue line at 455nm. It is about midway between three strong violets and three or four teal prominences. Next come moderately strong lines in yellow-green, two chartreuse, orange, a stripe of red, and a deeper red.
Next: Halogens