

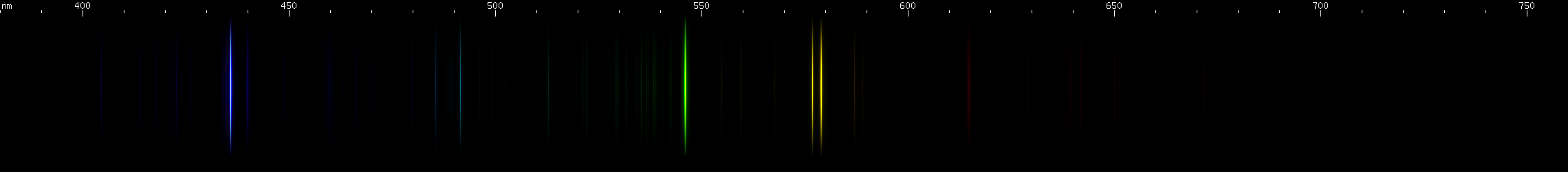









Now we get to some elements that look similar and are easy to confuse.
  |
  |
  |
  |
  |
  |
Sodium vs. Helium: In many cases all you will see is a bright amber line. When that happens, it can be very hard to differentiate these two elements. Sodium's yellow is at 590nm while helium's is at 587.6nm, making them very close to each other in hue and spectral position. Fortunately, humans are great at distinguishing shades of yellow (in technical terms, we have a wavelength discrimination minimum in this region, which is not a bad thing despite how it sounds) and it is possible to distinguish these two wavelengths by color, especially side by side. Nevertheless, if you can observe more lines than just the amber, it'll help you a lot. Helium is stronger in blue-greens and blues, and lacks chartreuse lines; sodium has stronger chartreuse than blue-green or blue. The two elements have very different patterns of lines in the blue-green and blue. Also, helium's red lines are more numerous than sodium's, and occur at a longer wavelengths. They appear much less orangeish. With helium, you may or may not be able to observe an extreme deep violet line, around 389nm. It almost doesn't show up at all in the photo, and is very hard to see with the eye because it's practically an ultraviolet wavelength, but it is one of helium's most intense lines.
Mercury vs. Krypton: This one's easy: krypton's yellow line is more orange than mercury's, and its green line is yellower. Krypton also has several bright blue lines, vs. mercury's single blue-violet 435.8nm line. While both elements have a moderately strong blue-green line, its position appears different: in mercury it is almost equidistant to the bright violet and green, while in krypton it's closer to all that blue. Finally, krypton has a pattern of orange and red that's missing from mercury entirely, and also does not resemble that red phosphor that's often added to mercury lamps.
Copper vs. Chromium: Not going to lie, these two look fairly similar. The biggest tell visually is that copper sppears to have three green lines, the brightest being on the redder end of the trio, while chromium appears to have four, with the brightest on the bluer end. Chromium's yellow line (actually a multiplet) has a different pattern of surrounding lines from copper's yellow lines, in fact chromium shows more moderately intense lines in general, while copper lines tend to be either intense or faint, particularly outside the blue-violet region. In the violet region, chromium has an intense triplet between 425-430nm, flanked by moderately bright lines on either side. Copper does not have quite the same pattern, and does not have any violet lines as strong as chromium's triplet.
  |
  |
  |
Holy mackerel! Is cadmium a doppelganger of hydrogen or of zinc? Well, there is a strong resemblance here, moreso than almost any other trio of simple few-bright-line spectra. The three can be distinguished by the color of their brightest visible line: for hydrogen it's red; cadmium, green; zinc blue. Hydrogen's blue-violet lines are faint while the two metals have strong blue lines. And zinc's blue triplet is close together, its lines all appearing more or less similar shades of azure, while cadmium's triplet consists of lines perceivable as two or three distinct hues.
Cadmium's resemblance to hydrogen is coincidental, but its resemblance to zinc is not, as we can see from the following comparison:

Arrows indicate analogous lines that originate from transitions in the same part of each metal's energy level diagram. As you can see, mercury fits the pattern too, although mercury's yellow line is actually three lines, but the brightest one is indeed analogous to the zinc and cadmium red lines.
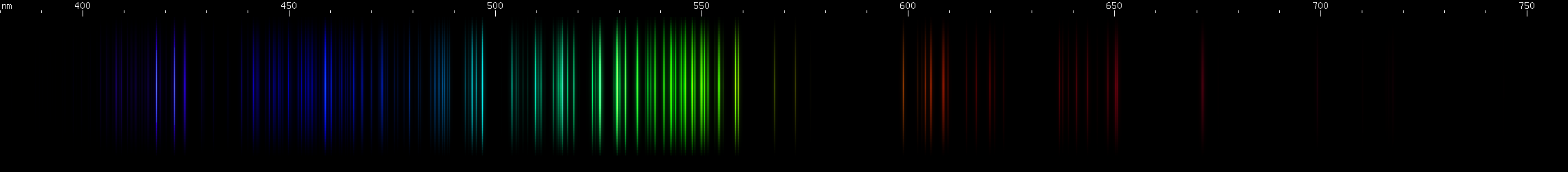
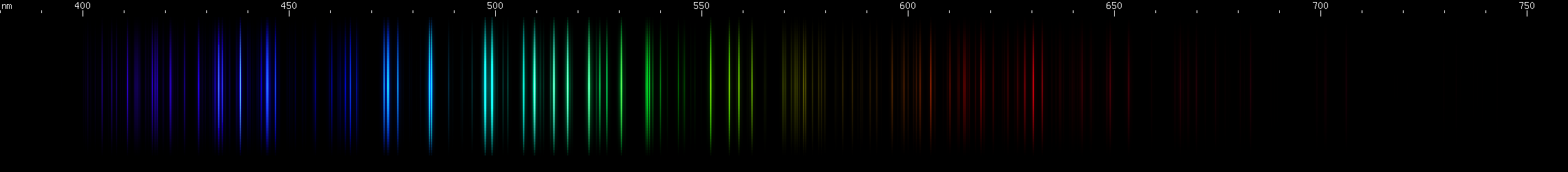
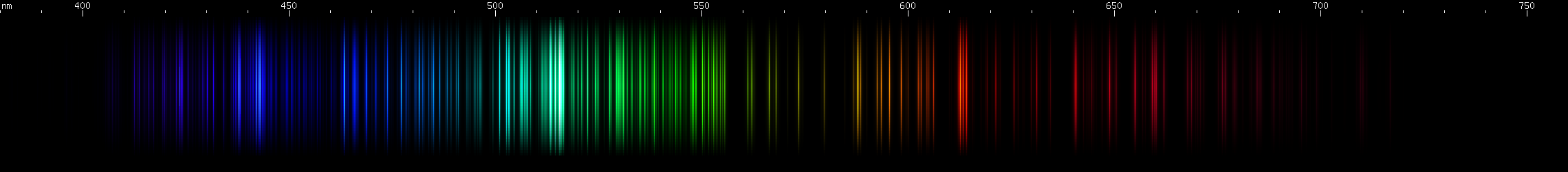
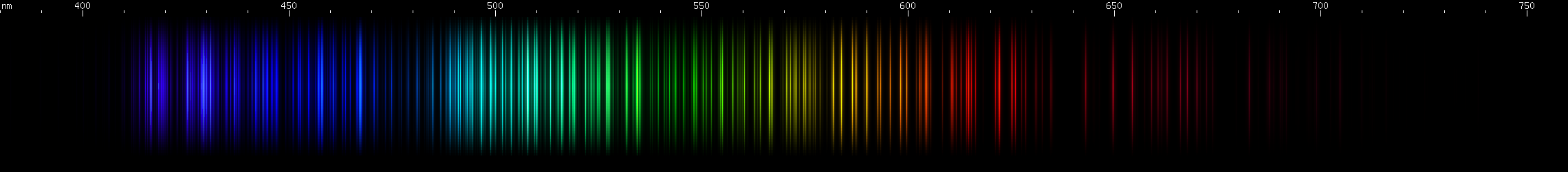
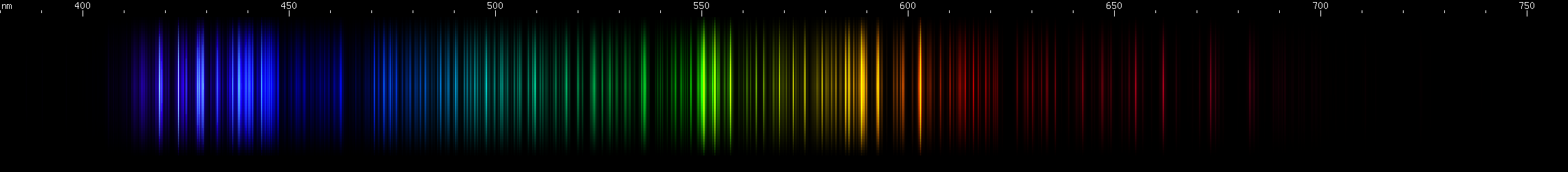
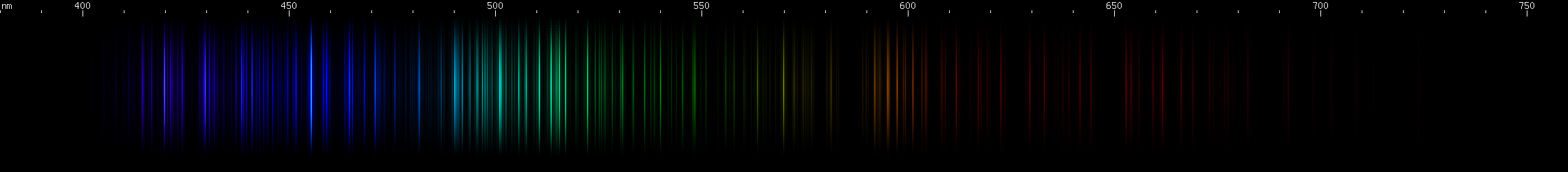
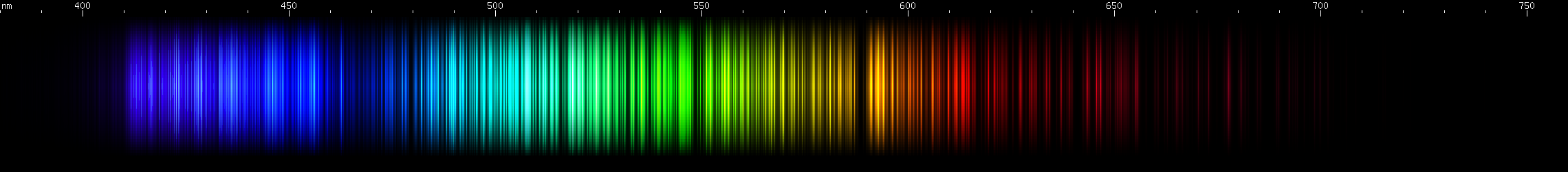
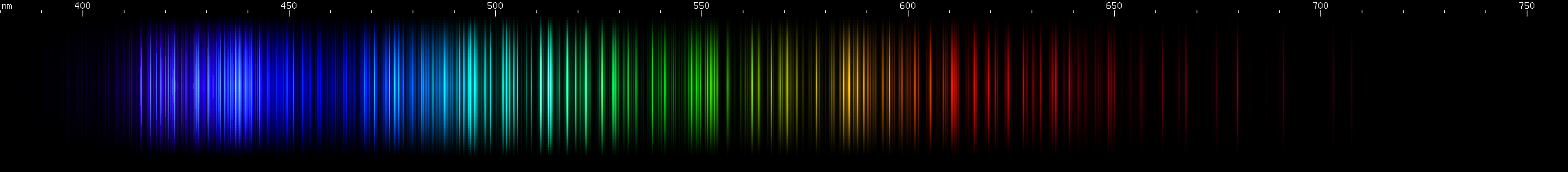
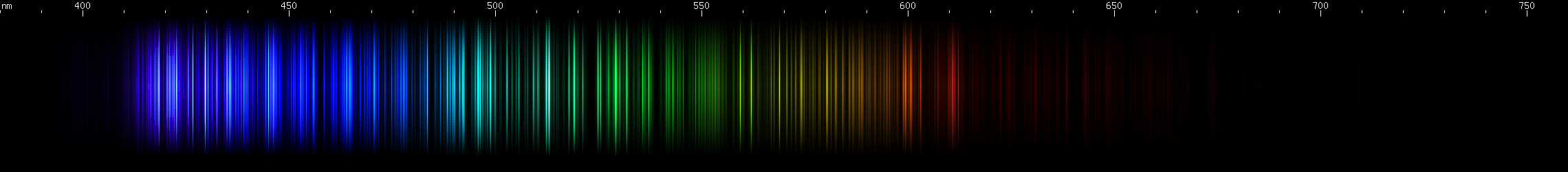
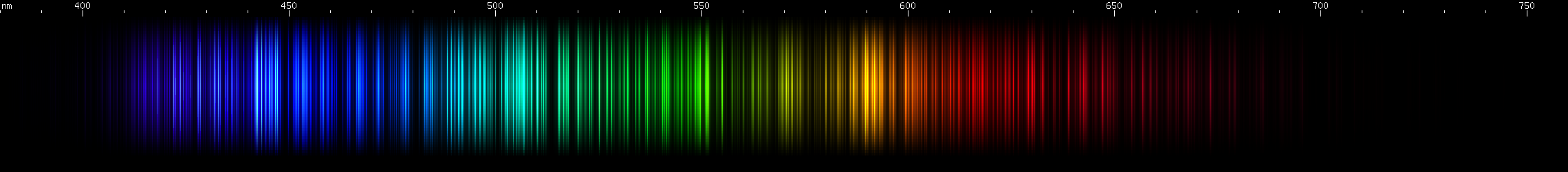
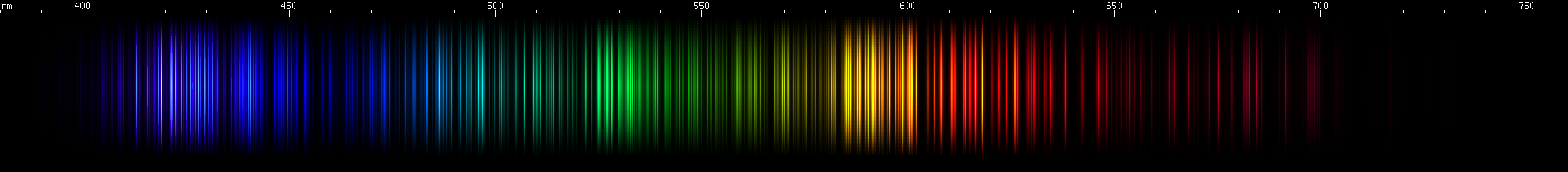
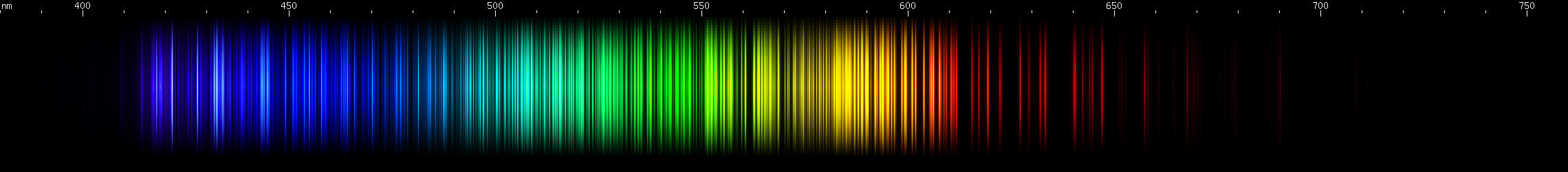
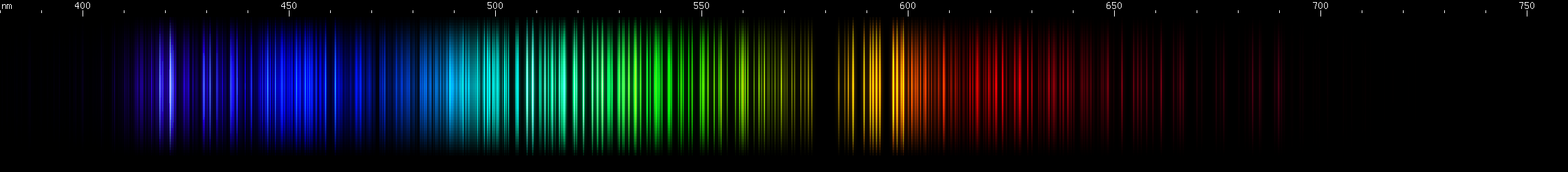
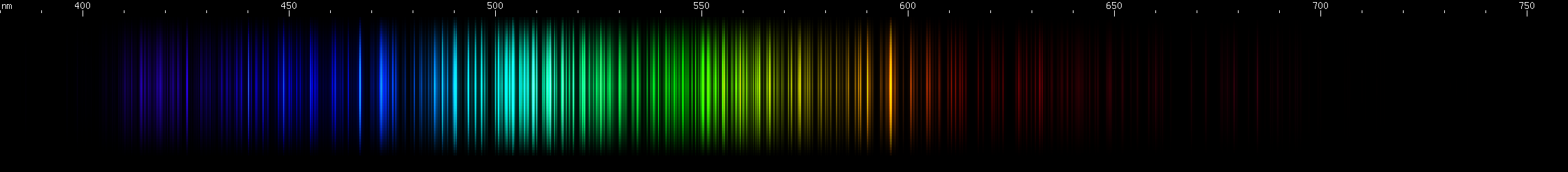
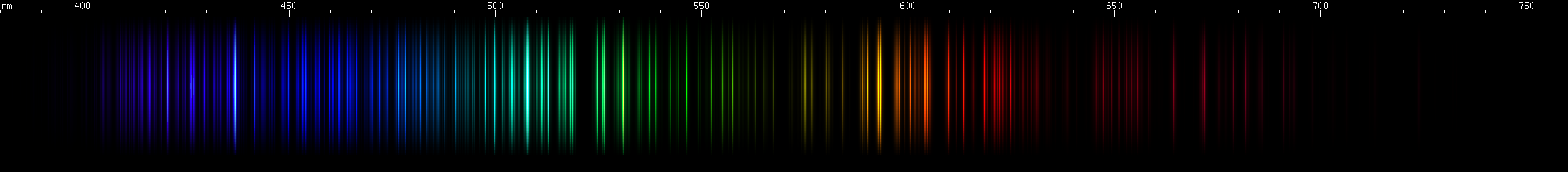
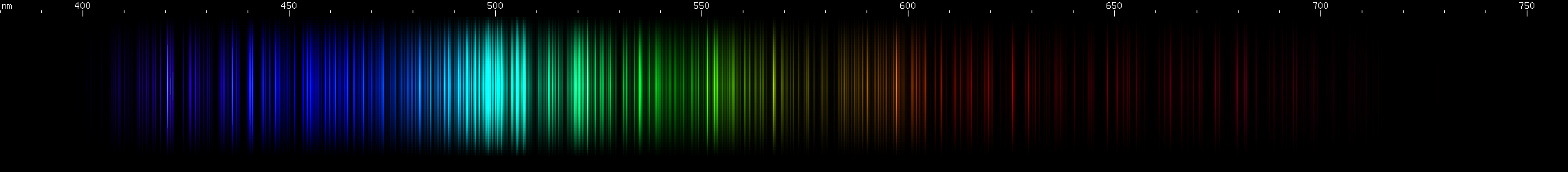
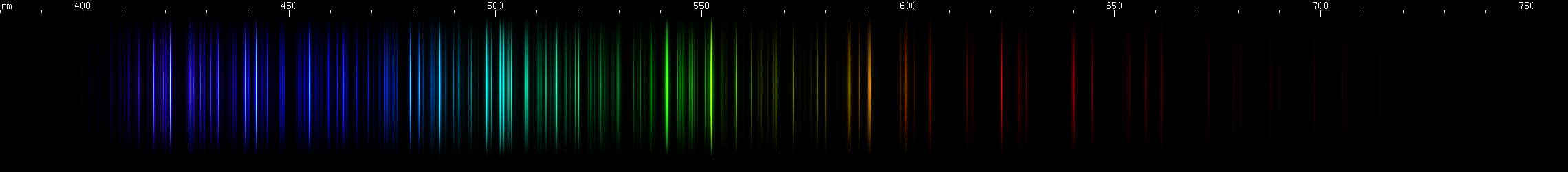
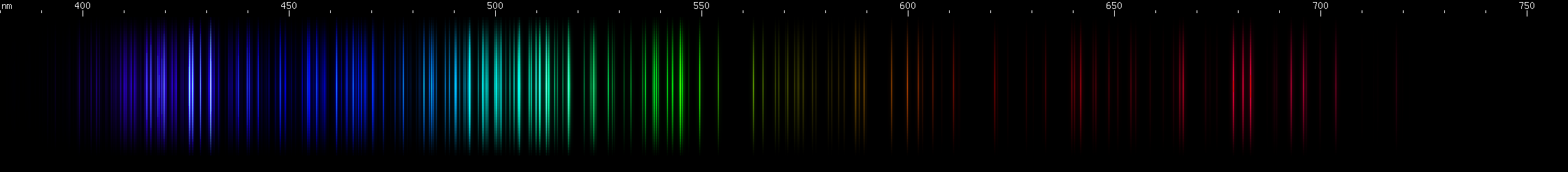
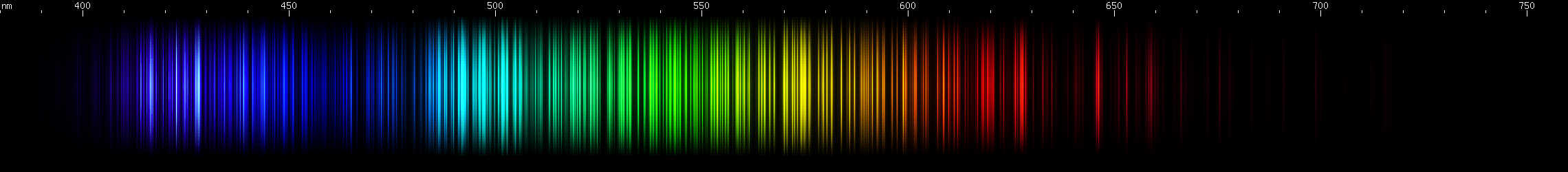
I promised you more Darned Many-Lined Spectra Dominated by Green and Blue, so here they are. Don't worry too much about learning how to distinguish them all just yet; there will be time for that later. For now, just know that they exist, and that telling them apart won't actually be as daunting as it looks.
Ready?
 |
  |
  |
  |
  |
  |
  |
  |
  |
  |
  |
  |
  |
  |
  |
  |
  |
  |
 |
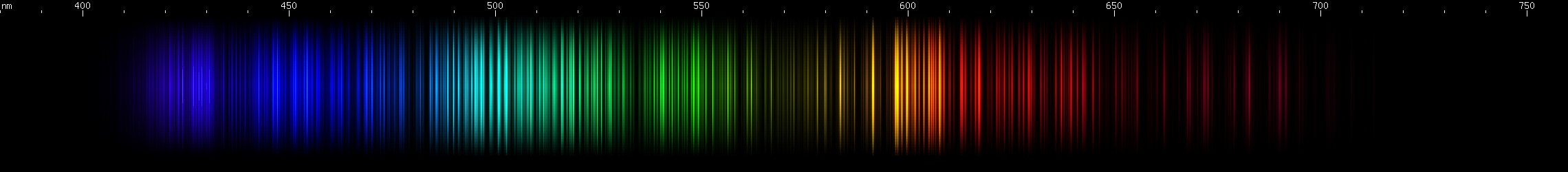  |
You can be forgiven for wanting to scream right now, but not to worry, each of the above spectra has distinguishing features that will be described later.