7.11 Chlorophyll
The chlorophyll molecule is based on a giant ring made of four smaller pyrrole rings connected by single carbon atoms, with the nitrogen atoms facing in toward the center. The entire tetrapyrrole structure is conjugated and planar, though it has non-conjugated non-planar attachments on the outside. The placement of the nitrogen atoms allows a metal to be chelated, with covalent bonds to two of the nitrogens and coordination bonds to the other two. The green color comes entirely from the conjugation of the large ring, and not at all from the metal atom, since magnesium compounds are white or clear.
Chlorophyll strongly absorbs blue light and moderately absorbs red light, causing our eyes to perceive a yellowish-green hue from the mixture of the remaining wavelengths. The photons that it absorbs give up their energy to the molecule, and that energy is then used to make sugars out of carbon dioxide and water.
There are actually a few different kinds of chlorophyll. The most important are chlorophyll A and chlorophyll B:
| Chlorophyll skeletal structures by David Richfield via Wikimedia Commons, under Public Domain/CC0. | |
In the near infrared region, just beyond the wavelengths we call visible light, chlorophyll does not absorb at all and appears white or clear. To perform its function, chlorophyll must be exposed to light of adequate wavelengths and sufficient intensity, so leaves try to get as much direct sunlight as possible throughout the day. All that sun can cause significant heating, so vegetation has evolved a defense; light that isn't used is reflected back, and quite strongly in the case of near infrared, in which foliage looks snowy, almost silvery. Leaves also reflect ultraviolet light, though not nearly as intensely, and insects look for this green-ultraviolet hue shift to find leaves, whether as food or as a place to lay eggs.
 |
 |
As a side note, I once saw a plant being grown indoors under a high-pressure sodium vapor lamp (an HPS lamp). This type of lamp takes advantage of sodium's ability to emit amber-yellow light when excited by an electric arc; the pressure spreads out the wavelengths of light so that instead of pure amber light you get a whole band of colors. It's great for illumination because these are almost exactly the same wavelengths our eyes are most sensitive to for waking hours tasks. The downside is that the light doesn't even come close to matching the absorption of chlorophyll, so the illumination was actually really inefficient for the plant:
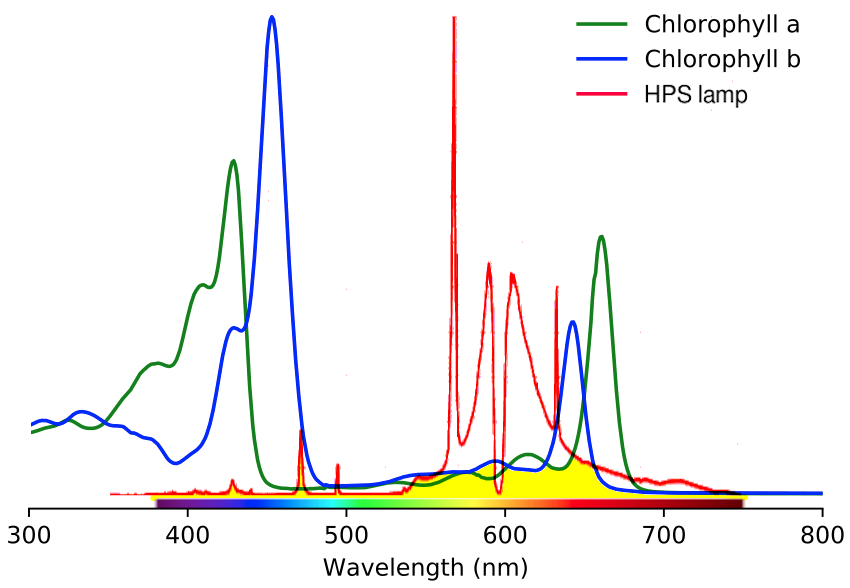
This graph shows the absorption of light wavelengths by chlorophyll (green, blue) and the emission of light wavelengths by a typical HPS lamp (red). Green plants can only use the energy where the lamp spectrum overlaps with the chlorophyll spectra (filled in with yellow in the chart), reflecting back all the rest of the lamp's emitted energy unused.
Chlorophyll spectra from Serge Helfrich, CC BY-SA 4.0, via Wikimedia Commons.
HPS lamp from LMRoberts at English Wikipedia, CC BY-SA 3.0, via Wikimedia Commons.
Combined image licensed under the Creative Commons Attribution-Share Alike 4.0 International license.