


Now we come to the remaining handful of stable elements, and a couple of unstable ones. The densest substance on Earth is in this list, along with the highest melting point and the only primordial fissionable nuclide.
  |
  |
  |
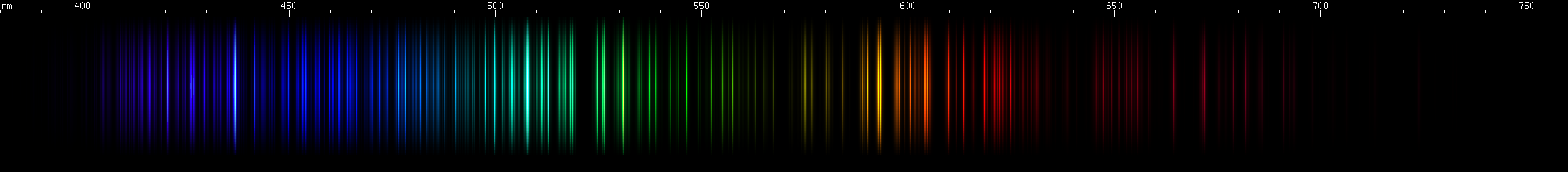
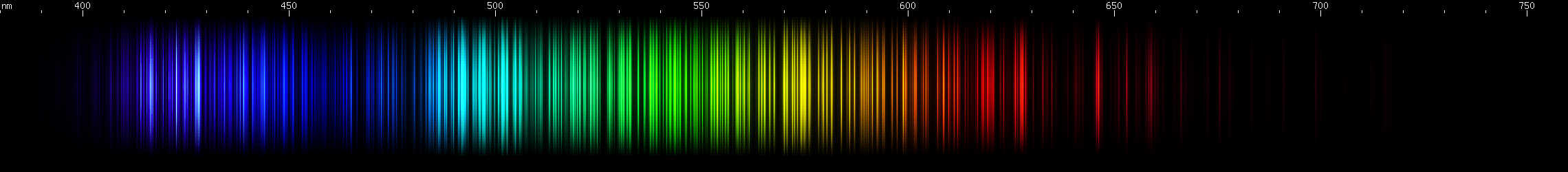
Hafnium: Hafnium has a region of intense emerald-green lines, roughly 505-520nm. Greens of longer wavelengths are less intense, although secondary brightness is apparent in cartreuse hues, approximately 565-570nm. A blue stripe (near 465nm) sits near a pair of indigo bright spots (near 455nm) then after a gap there's a pattern of deep violet lines. Some amber and orange-red are visible, especially near 605nm, and then a less intense red clump, as well as a strong deep red line all the way out out near 665nm.
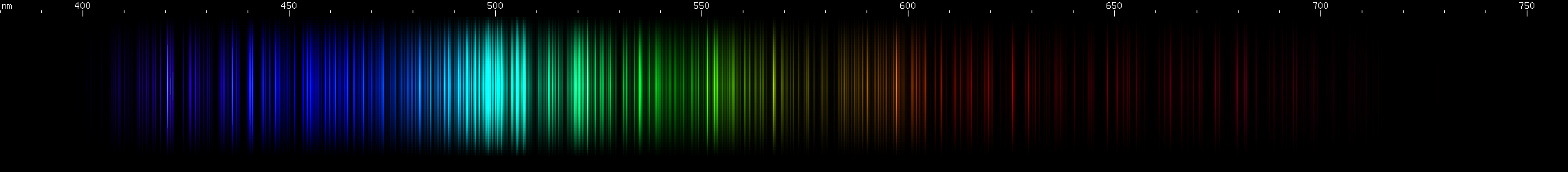
Tungsten: Strongest lines in the cyan region near 500nm, falling gradually dimmer towards longer or shorter wavelengths. Next to this peak is an only slightly lesser emerald peak, and then another yellow-green peak further out (near 550nm). The sequence of nearly steady greens ends with two very close together yellow lines (~580nm), after which two orange clumps are apparent. The red region contains many progressively weaker lines. Two mild bright spots appear in the indigo region (near 460nm), followed by two brighter spots (near 440nm), and then past those are some even brighter violet lines.
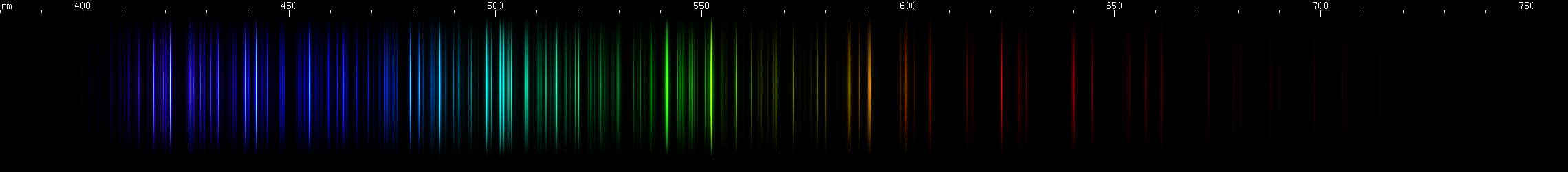
Osmium: Two moderately intense violet regions with a gap between them at 422nm. Note that iron and iridium have visually similar gaps. Next are the two brightest lines, indigo near 440nm, close enough together to appear to blend; then there's a 455nm bright line. Note that ruthenium has a strong line at this wavelength too. Osmium has azure lines building up in "steps" to a teal peak around 502nm, then tapering somewhat through the emerald region. Three yellow-green lines stand out, the middle one brightest (~552nm) and a bit longwave of center for the three. Then there are two yellow lines and a brighter orange, almost equally spaced. Some moderate to faint red lines can be seen: one then two, a space, one more, a larger space, and then a deep cherry-red line.
  |
 |
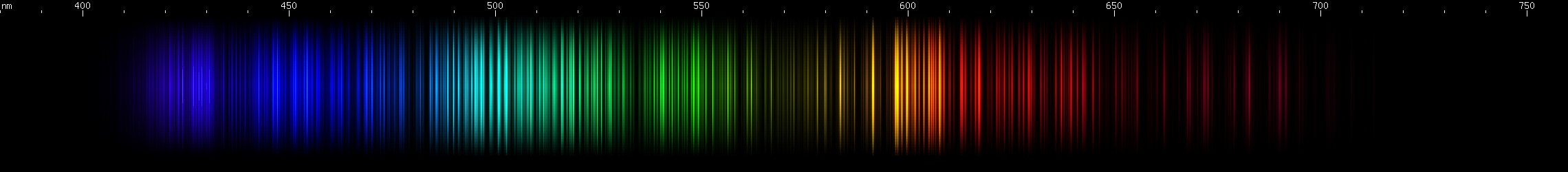  |
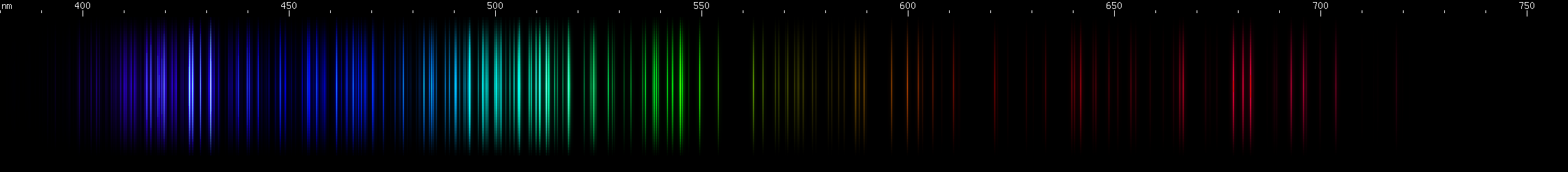
Iridium: A similar violet feature to osmium, with the gap in the same place, but notice that the part between the bright area and the gap is clearly two separate bright zones, not just one. There are a few moderately weak blue stripes, brightest in the middle, and then a long stripe from cyan (~485nm) to chartreuse (>550nm), brighter at each end and in a grass-green area near 540nm, and brightest around 510 emerald green with a slightly less intense peak on either side. Yellow and orange clusters of lines can be seen. The spark experiment was very difficult to carry out because of the sample size, but the photo shows several deep red lines in the 670-700nm region, that do not resemble any possible contaminant.
Thorium: I don't have a photo of thorium, but not for lack of a sample. It is simply too heavily oxidized. Thorium's visible spectrum supposedly resembles tungsten, but with brighter reds and a gap between the bright cyan and blue/violet regions.
Uranium: Bright orange lines (595-610nm) and an intense cyan/green stripe (485-530nm) dominate uranium. The orange brightness is followed by reds tapering off gradually over a large range, breaking into localized spots towards the end. Some moderately strong yellow-greens can be seen in the 548-562nm region. In the blue-violet there are a couple of moderate bright regions followed by a wide violet swath around 410-430nm.
Next: Conclusion