


Collectively known as the rare earths, these elements are actually not all that rare. I am not including promethium as I do not expect to ever photograph a spectrum of so intensely radioactive an element; exposure to even one gram could be immediately hazardous to health. We'll look at all the lanthanides except that one, and their lighter congener yttrium, starting with the easiest to recognize.
  |
  |
  |
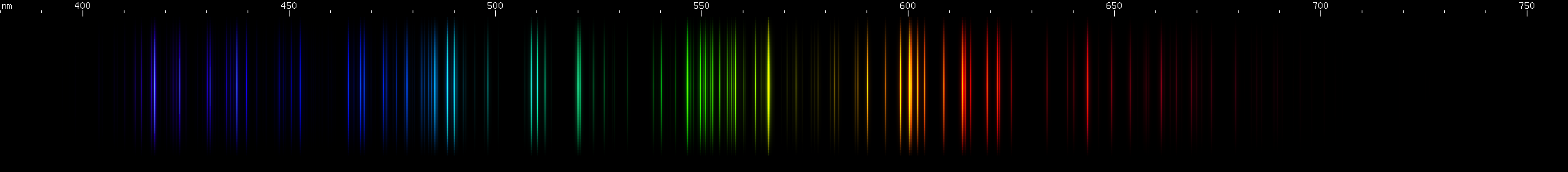
Yttrium: Bears a resemblance to scandium, but with yellow-greens less chartreuse and reds more orange. Yttrium's visible spectrum is dominated by small intense orange and aqua brightnesses. Amber and orange-red occur on either side of the bright orange, and there's a discrete red line. The yellow-green lines (~540-565nm) form a pattern of a central stripe stripe with a separate line on each side, the yellowest line brightest. Three emerald green lines occur, the middle line dimmer and very close to the line on the bluer side of the trio. In the violet region are four lines, as two groups of two, the outermost lines brightest.
Ytterbium: Not to be confused with yttrium, ytterbium somewhat resembles its soundalike. The most obvious difference is that ytterbium lines are more spread out, and in places seem to be almost evenly spaced. Yttrium's distinctive green stripe structure is missing, but ytterbium's greens themselves make a distinct pattern, with a central bright spot (~534nm) flanked by two lesser bright areas, then flanked off center by two more brightnesses around 555nm yellow-green and 508nm teal. Three bright lines can be seenin the yellow region, between 570-590nm, and then an orange pair stradling 600nm. The reds continue the sequence, culminating in three bright red lines between about 649nm and 680nm. In the blue-violet, the pattern continues again, including prominent aqua (493nm), violet (near 420nm), and almost halfway between them a blue brightness (near 460nm), amid other intermediate moderately bright areas.
Lutetium: With lutetium we see several discrete bright lines: two red; one orange; one grass-green; a few emerald green; a few teal; two blue, bright then dim; to violet, dim then bright. There is also a less intense yellow line almost halfway between the orange and grass green.
Some of the lanthanides have blue-heavy spectra, where the most intense lines are in the deep violet, but progressively less intense lines occur in the blue/green, yellow, and red regions.
  |
  |
  |
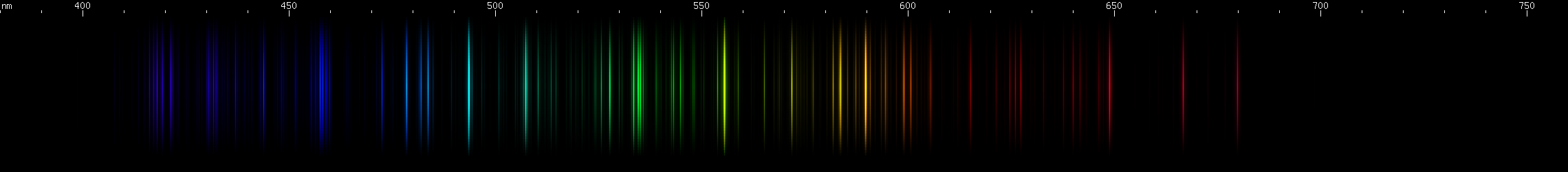
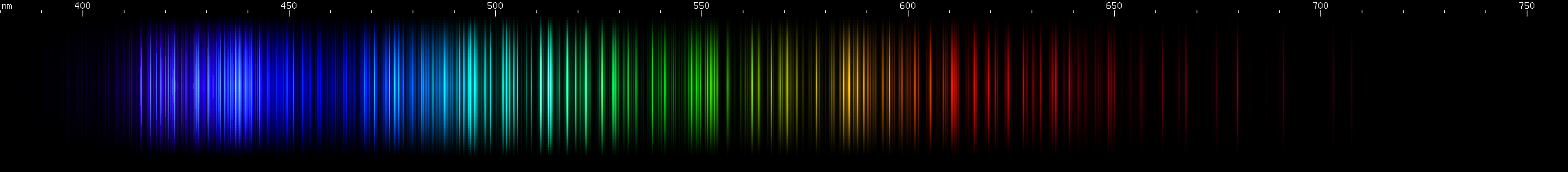
Lanthanum: Lanthanum is characterized by aqua and emerald stripes, with dimmer stripes flanking the aqua, and the emerald stripe wider and brightest at the redder end. A stripe of indigo-blue also tends brighter towards the longwave end, except for a single bright line at the shortwave extreme. This is separated by a rather large gap from a detailed violet stripe containing two smaller gaps. We see several moderately faint stripes throughout the green region, and two moderately bright red stripes, separated by another decent sized gap.
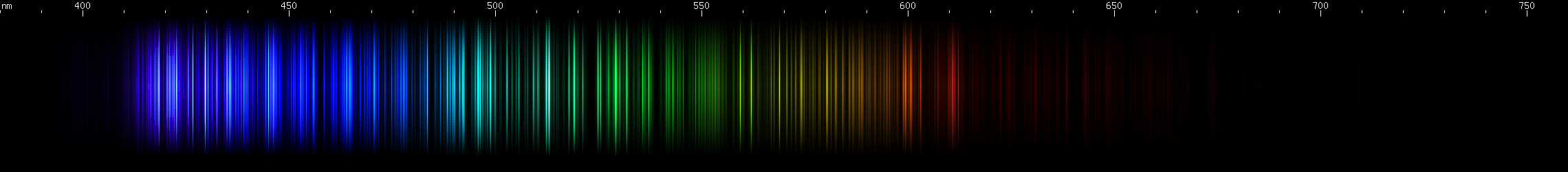
Praseodymium: The strongest lines form a sort of violet block, with two bright lines close together on the shortwave end, one on the longwave end, and a sort of "webbing" of lines between them. Not many obvious features (a faint brightening near 460-470nm) until the sequence of bright zones from aqua to pure green, forming a pattern of dim, bright, dim, two bright, then two groups of two. Lesser bright zones occur in the yellow-green region, as well as some amber through orange-red.
Neodymium: The most intense deep violet lines look more indistinct, although this could be the fault of the photo. Two groups of two can just be made out in the indigo and blue, against the background of moderately intense lines. A cyan brightness and a green brightness can be seen, with almost an inverted bell curve of brightness between them, followed by three more green lines or narrow stripes. A little bit of faint yellow can be observed.
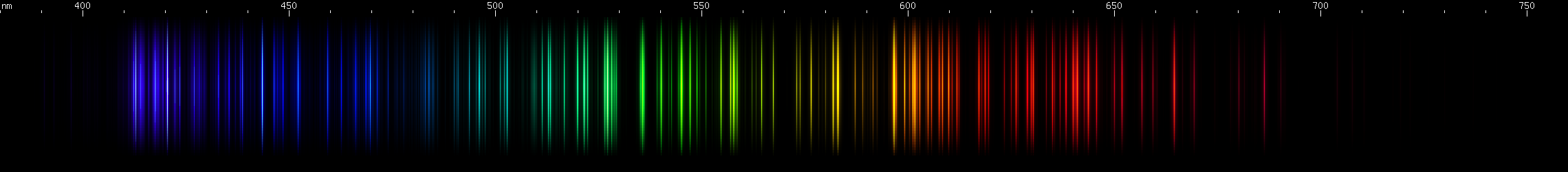
We have a near lookalike among the lanthanides: europium, of all things, resembles carbon, but with strong violet lines.
  |
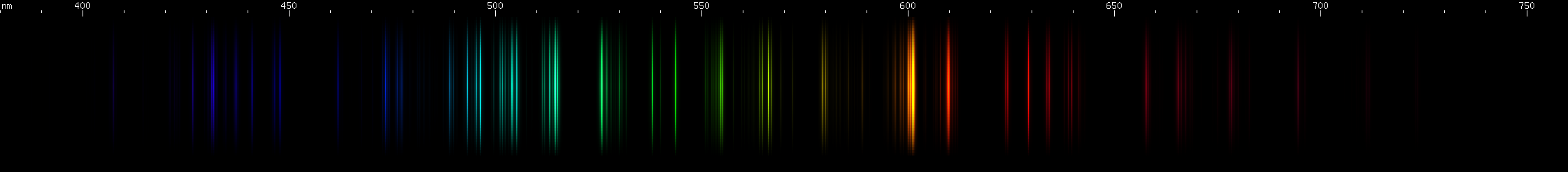  |
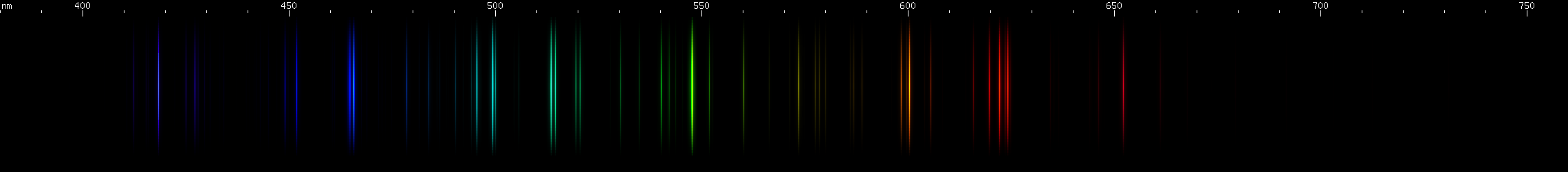
Carbon's lines are sparser with less yellow and red, and dominated by its tight 600nm orange multiplet, while europium has a deep violet (390-397nm) triplet that carbon lacks. Europium's blue/violet pattern of bright spot, bright spot, bright stripe is also missing from carbon.
Some of the lanthanides emit large numbers of lines in the green and blue regions. Here's how to distinguish them.
  |
  |
  |
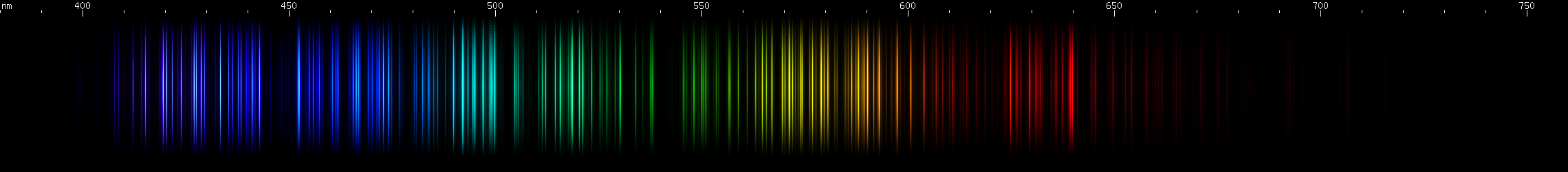
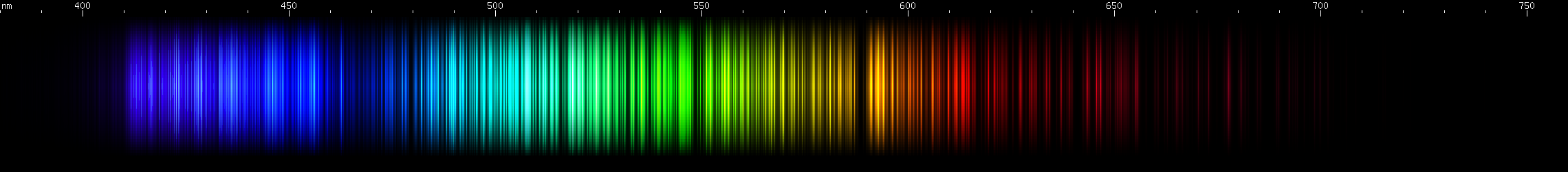
Cerium: The blue/violet lines look like a solid, not quite featureless, block. Up to eight bright spots can be made out in the photo, in a specific pattern. There aren't any particularly bright lines in the blue region. In the green, a sequence of close together bright spots can be made out, fading out into the red region. A rather wide zone of mild brightness occurs in the orange-red, and at longer wavelengths a faint cherry-red bright spot.
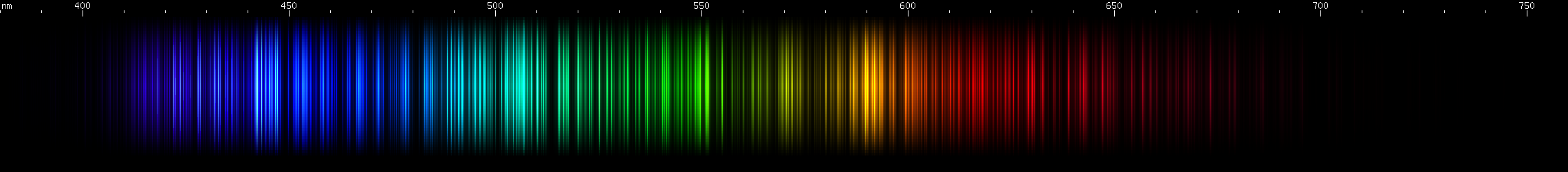
Samarium: Has a bright indigo stripe (~440-450nm) with more indigo trailing off to longer wavelengths. Aqua and cyan builds up to two strong teal-emerald brightnesses. Lesser bright zones occur in the yellow-green and amber. Finally, there are many indistinct red lines of moderate brightness.
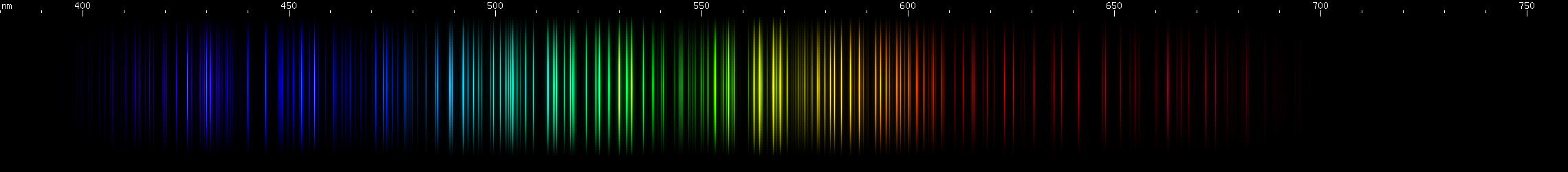
Holmium: The visually brightest part is in the chartreuse region (~560-570nm), while awide span of greens can be seen tapering off into cyan. There is also a moderately bright amber region. Some reds can just be discerned, including a line near 640nm and a few deeper red lines. In the blue-violet regions, a pair of lines near 440nm and 444nm are flanked by lesser violet and indigo prominences, then there's a pattern of deep violet.
Some of the lanthanides seem to show increased brightness in or near the orange part of the spectrum.
  |
  |
  |
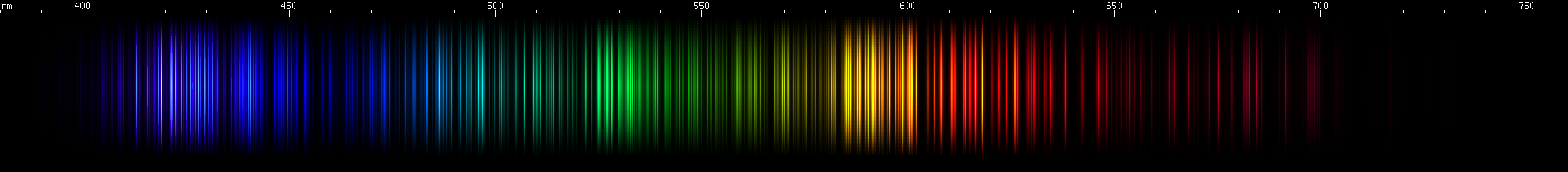
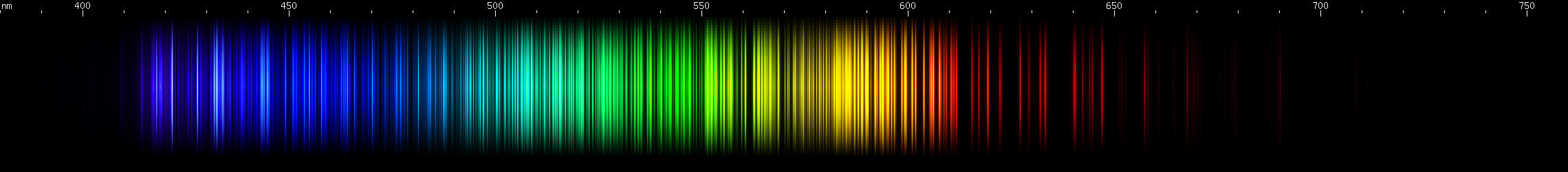
Gadolinium: By far the brightest visible lines are in the yellow-orange-red region, about 580-630nm. The green region is strongest in the middle, around 530nm, with also some blue-green and chartreuse. A pattern of violet stripes is distinctive: two or three, then one, then one more, then three, then one with one or two more tapering off into deep violet.
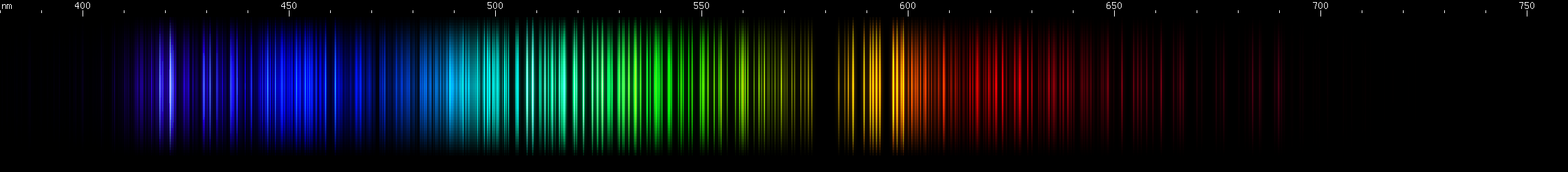
Terbium: Brightest lines in the yellow, fading into orange-red, and three clumps in the violet. Greens are strongest from grass-green to yellow. There are a few bright spots from emerald to azure. The blue/indigo region looks relatively featureless.
Dysprosium: A narrow amber-orange prominence is accompanied by a dimmer orange-red fadeout and some dim yellows. Greens are strong from cyan to grass-green, with particular bright areas near 535nm and 550nm. Blues feature a wide indigo stripe. Bright violet near 420nm, may be resolvable as two clusters of lines.
Erbium and thulium resemble each other rather closely.
  |
  |
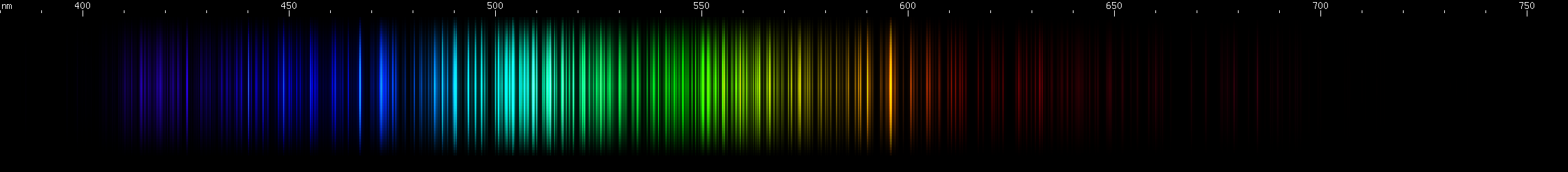
Erbium: Erbium's greens occur in two wide groups: one more teal and one more chartreuse. Erbium has a red stripe with a gap near the middle, then another red stripe most intense at the extremes, then a pair of deep red lines (near 680nm). In the blue region there's a pattern of dimmer, brighter, dimmer, two or three brighter, space, then another brighter. Then come four almost evenly bright, almost evenly spaced violet lines.
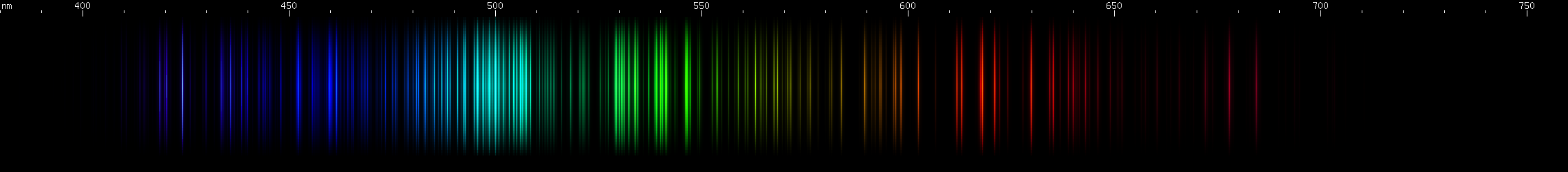
Thulium: Thulium, similarly to erbium, has its green lines concentrated in a cyan/teal group (centered near 500nm) and a yellow-green group. However, the two metals have different patterns of red and blue. For blues, there is a pair of indigo bright spots, one diffuse near 460nm and one sharper near 450nm, with a lesser brightness between them. A group of two or three moderately intense violet lines follows, then one line, then a pair of intense violets, the shortwave member appearing double. Two more violet lines (actually close pairs of lines) follow, although they may be too faint to see. Thulium's yellow and red lines seem to vary a lot depending on the amount of ionization in the excited sample, but generally it shows more red than erbium does.
Next: Last but Not Least