


It is almost impossible to get a good photo of a halogen spectrum. Spectrum tubes are available for chlorine, bromine, and iodine, but they all suffer from problems. A chlorine tube emits a series of molecular bands superimposed over what appear to be atomic lines; the molecular bands have to be masked out. A tube advertised and sold as bromine emits strong N2 bands, unless by some strange coincidence molecular bromine looks exactly like molecular nitrogen and not at all like its congeners. An iodine tube emits some nice blue-violet iodine lines, and then a wide continuum from green to red, presumably a molecular band, that almost completely drowns out all lines and has to be digitally processed to recover the spectral features. Worst of all, even when an iodine tube is lit up for less than the recommended 30 seconds on/30 seconds off duty cycle, the tube nevertheless dims after just a few cycles and quickly stops working altogether. Fluorine tubes are not available as this gas is too reactive and cannot be kept in glass. Probably for the best since fluorine would find some other way to disappoint.
  |
  |
  |
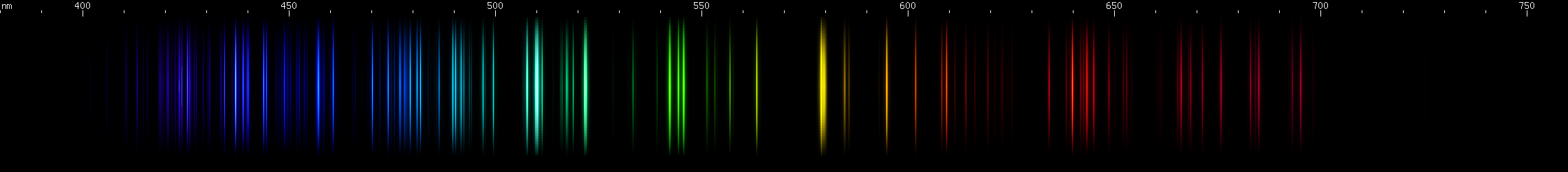
Chlorine: Several red lines and a pattern of yellow (579nm) flanked by orange and chartreuse. Bright greens near 543nm and 510nm. The azure lines form a rounded looking clump with a gap near the middle. The remaining blue lines form 4 groups between 400-460nm. Each group's brightest line seems to be the one closest to 440nm.
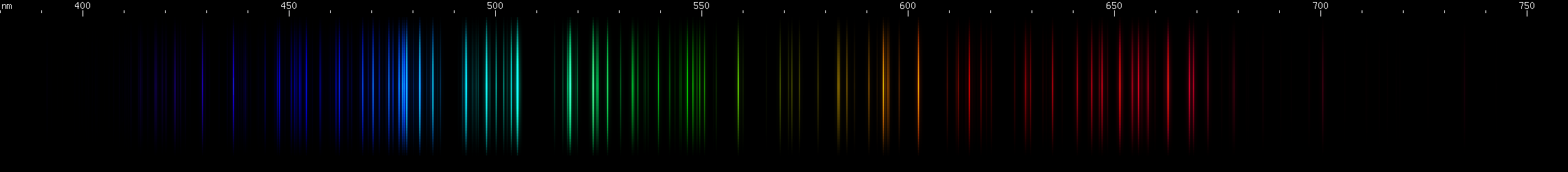
Bromine: I did my best to remove nitrogen bands from the photo, while keeping as much bromine light as possible. Bromine seems to give strong blue-greens with some azure, orange, and red; and less intense grass green and violet.
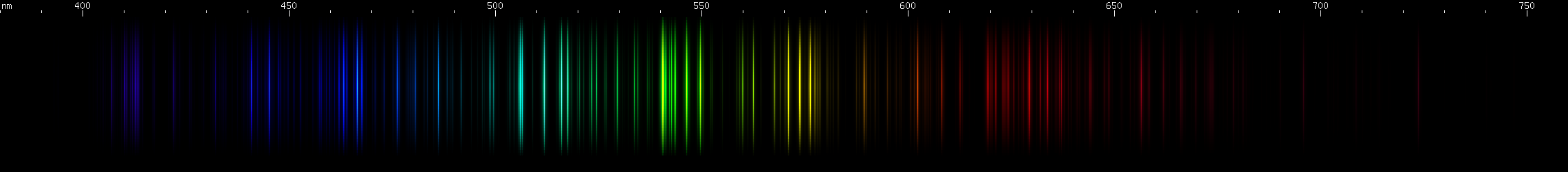
Iodine: The green lines between 540-550nm are probably iodine's visually brightest feature. The pattern of 3 close together 570-577nm yellow, several bright 540-550nm green, several ~500-520nm teal, and ~410nm violet, is the charactristic pattern of iodine. The blue lines also form a subtle but recognizable pattern.
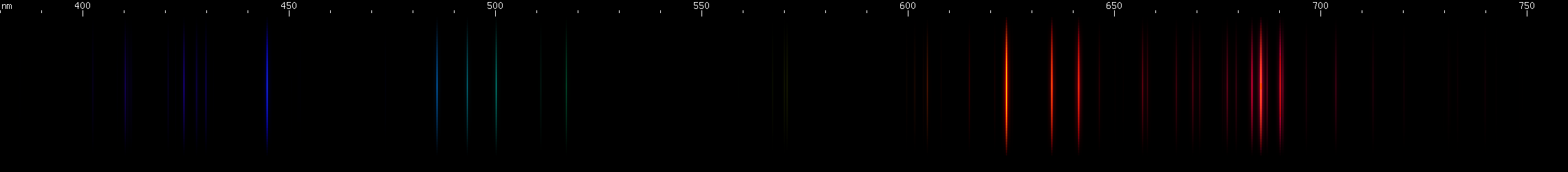
Among the halogens, there is one lookalike.
 |
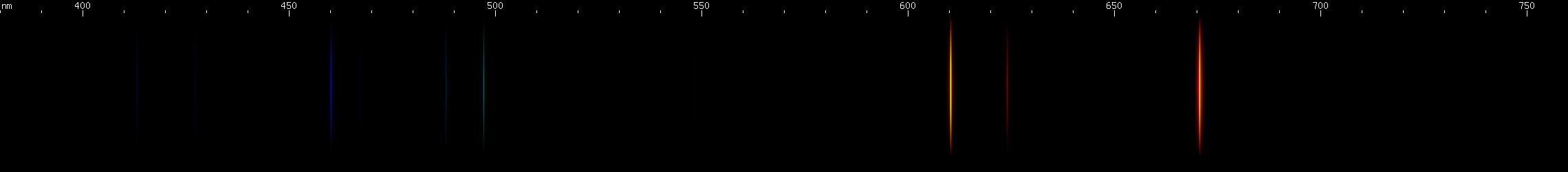 |
Although it has not been possible to photograph a fluorine spectrum, its Grotrian diagram indicates that the red lines would indeed be the strongest.
Next: Transition Metals