  |
  |
  |
  |
We've already seen magnesium, and we know that its bright green triplet is analogous to triplets in zinc, cadmium, and mercury. All of these elements in their neutral form have two-electron spectra, and share a structure of energy levels divided into singlets and triplets. The 3S and 3D series seem to be particularly intense, with the former usually occurring in the visible region. Helium is another two-electron spectrum, although it has a few differences from the metals. Seemingly out of nowhere, another two-electron element is the rare earth metal ytterbium. Its heavier congener nobelium is predicted to also fit this category, however nobelium's spectrum is unknown.
  |
  |
  |
  |
I don't have a photo of radium, but you can see its lines from my source data in the interactive viewer.
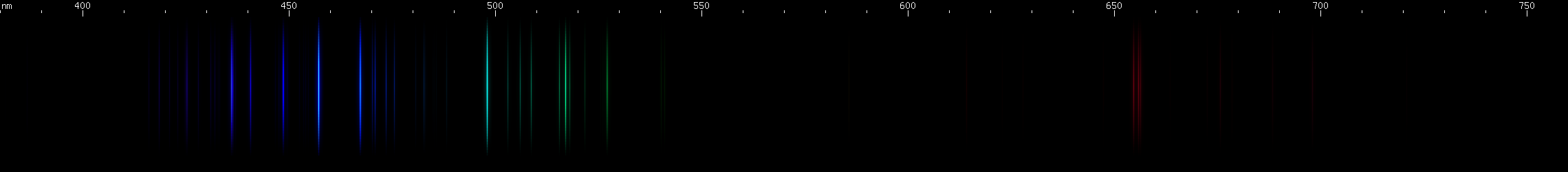
Beryllium: A specific pattern of bright green and moderate blue, combined with a deep red (almost 700nm), identifies beryllium. You know, my sources both said it would give strong blue lines, an intense blue spark. So I procure a sample, and I'm using a tool to pull these super light chunks of metal out of the container, and I'm excited to see how blue their sparks will look. And I hook up the electrical connections, and ready my diffraction grating, and okay here goes... and imagine my surprise when all this green jumps out at me. Well, that's why I check as many of these elements as I can.
The remaining alkaline earths are very reactive and flammable, so it was necessary to exercise caution when obtaining their spectra. The metal samples of calcium, strontium, and barium, when sparked, all emitted a pale bluish light along with some bright colorful embers as the metals attempted to ignite: orange for calcium, red for strontium, and greenish yellow for barium. These bright colors arise when the metal oxidizes, and then the oxide emits molecular bands from CaO, SrO, or BaO. Such molecular bands are used in fireworks; strontium's red flame and barium's green flame happen partly because of molecular bands, and even copper salts in pyrotechnics produce blue bands in contrast to the free metal's green spark.
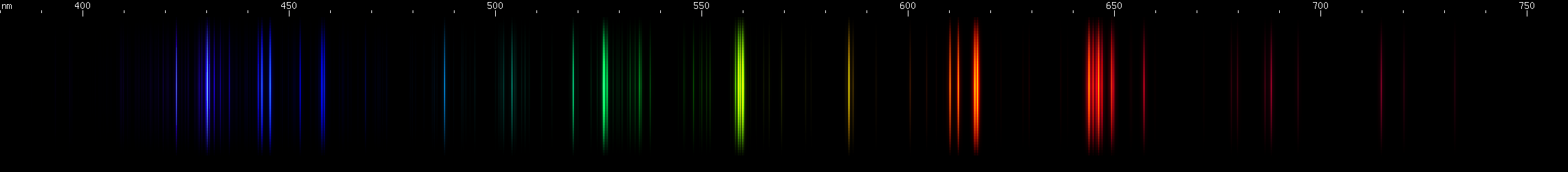
Calcium: A pattern of strong red, green, and violet lines identify calcium. In a flame test, calcium produces reddish orange. To identify atomic calcium, notice the intense clusters of lines near 615nm orange-red and 560nm chartreuse, plus additional bright regions near 645nm red and 525nm green. Combined with the fainter yellow 586nm line they form a visually even spacing. A far red line appears as a distant outlier to the spacing pattern. Calcium also has its own pattern of blue-violet: moderate, dim, bright, dim, two bright.
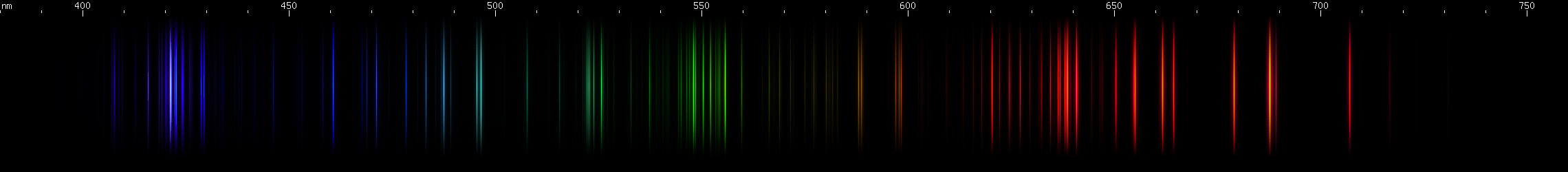
Strontium: Notice all the intense reds, over an unusually wide range, that also appear in its flame spectrum (the extra glow underneath the spark lines in the photo). See also the pattern of greens and green-blue, which is distinctive. The violet pattern differs from that of calcium.
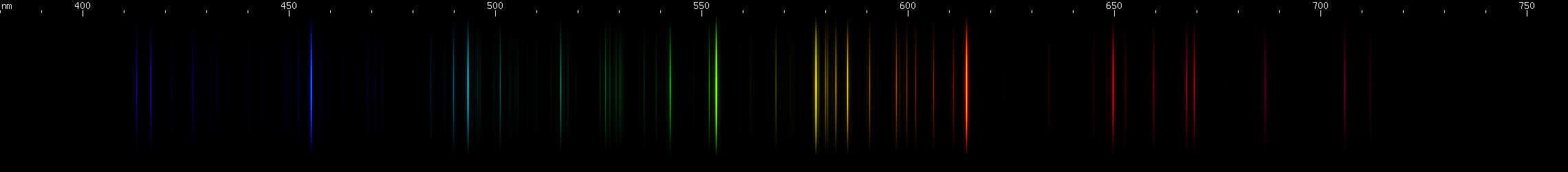
Barium: Though famous for its apple-green 553.5nm line and its apple-green flame test, the spark was not green and revealed brighter lines than that one. There's a pattern of yellow through orange-red and then cherry red that almost recalls a similar pattern in oxygen, except spread out over a wider range of wavelengths. (If you like, you can compare them here.) A single moderately intense green line occurs next to the 553.5nm line, and then a few more rather faint greens, instead of there being two separate bright zones. But probably the most interesting part of barium's spectrum is the Ba II doublet at 493nm and 455nm. Recall that neutral cesium (Cs I) has its P-alpha doublet in the infrared. Ionized Ba II emits analogous lines to Cs I, and these two lines in particular are Ba II's P-alpha doublet. So while one element's most intense doublet is invisible to our eyes, we can see that same doublet made visible in the next element, in a region with basically no interference from Ba I lines.
Just for reference - not important to memorize anything from this table - wavelengths in nm of some important lines of two-electron spectra:
| Element | 1P | 1S <- 3P (forbidden) |
3S | 3D | 1D |
|---|---|---|---|---|---|
| Helium: He I | 58.4 | 59.1 (faint) | 706.5 | 587.6 | 504.2 |
| Beryllium: Be I | 234.9 | 454.9 | 332.1 | 249.5 | 698.2 |
| Magnesium: Mg I | 285.2 | 457.1 | 516.7-518.4 | 383-384 | 880.7 |
| Calcium: Ca I | 422.7 | 657.3 | 610.3-616.2 | 442.5-445.5 | 585.7 |
| Strontium: Sr I | 460.7 | 689.3 | 679-707 | α: 2603; β: 483-497 | 655.0 |
| Barium: Ba I | 553.5 | 791.1 | 719-790 | 422-440 | 821 |
| Zinc: Zn I | 213.8 | 307.5 | 468-481 | 328-335 | 636.2 |
| Cadmium: Cd I | 228.8 | 326.1 | 468-509 | 340-361 | 643.8 |
| Mercury: Hg I | 184.9 | 253.7 | 404.7, 435.8, 546.1 | 297-366 | 579 |
| Ytterbium: Yb I | 398.8 | 555.6 | 680, 770 | 458, 494 | ? |