
How easy is it to use spectroscopy to identify elements in a mixture? Well, you have a list of wavelengths, and next to each wavelength is an element symbol, and that tells you what material could be present that emits that line. So you dial in a spectrometer, find some emission lines, look up their wavelengths in the tables, and then you have your answer. Or better yet, the spectrometer is computerized, and it does the lookup for you - you get just the results, without ever having to look at a spectral line. That's how it's done, right? But is that the only way? Can spectroscopy be done without a multi thousand dollar piece of laboratory equipment? Can someone analyze a sample, such as an alloy, with the naked eye and a diffraction grating, by recognizing the patterns each element produces? Yes, yes you can.
Case in point, here's a spectrum from a piece of mystery metal that I was asked to analyze:

Immediately it can be seen that zinc dominates the spectrum, with some copper lines as well:
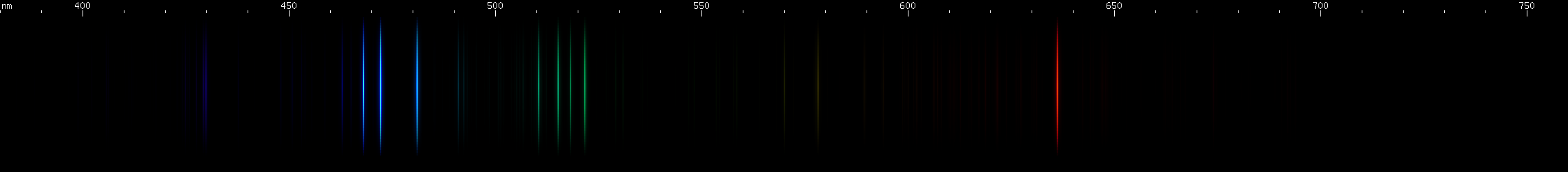
However, some other element had to also be present. I consistently saw what looked like a single blue-green line in between the bright copper and zinc "triplets" (only the zinc one is a true triplet), a line that didn't belong to either element. By noting its position relative to the known Cu znd Zn lines, I pinned down its wavelength as roughly 499nm.

I also noticed a yellow-green line that was almost as far again past the bright Cu lines as the bright Cu lines are from the bright Zn lines. It was about midway between the Cu 522nm line and the Cu 570nm line.

To identify the mystery third component of this alloy, I would have to look for what elements have strong lines near 499nm and ((22+70)/2 = 46) 546nm. So I loaded up the interactive page for Zn, added Cu to the view, and adjusted the relative amounts to look like the photo, about 2.5:1 Zn:Cu. Then I began adding and removing elements one by one looking for the right lines. Bismuth? Antimony? Lead? Tin? No, none of these were right.
After much searching, I finally found a metal with lines in the right places. I finally had the identity of the mystery element. Keep in mind I have no expensive spectroscope; I'm doing this with a 12V circuit and a diffraction grating. The naked eye view of the spectrum was quite a bit clearer than the photo, but still I was limited by my DIY setup and low budget apparatus.
The identity of the mystery element now known, and confirmed by adding it to the onscreen mixture and seeing that the simulation matched my observation, I then added a clickable how-to-identify instruction, specifically "look for the 498nm and 547nm lines", to its interactive page. Then I sought to create such instructions for all the elements whose visible spectra are known, that way it will not be necessary to go through so much trouble.
The third element in the alloy was nickel. The mystery alloy turned out to be German silver, a nickel-zinc-copper combination that contains no actual silver. This made sense given that this sample used to be a spoon that had been melted down.
In this way it is possible to cheaply identify the components in a mixture, qualitatively although generally not quantitatively.
Another time, I was photographing the spectrum of a metal halide lamp in order to use the spectrum on this very website. The most common type of MH lamp, the kind that produces an intense white light, is based on mercury and sodium vapors with a scandium halide added. Hg and Na together produce a stale beigey-white glow, but the Sc adds blue-green, blue, and orange-red lines that help balance out the perceptual color of the lamp's illumination, as well as the colors of objects lit by the lamp. Naturally, I expected to see only lines of Na, Hg, and Sc, but when I compared that mixture in the interactive page with the actual photo, I saw extra lines of an element that took me a moment to identify.
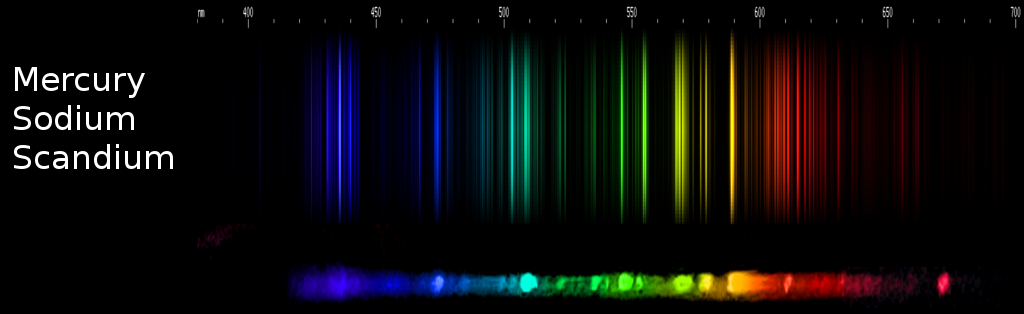
Fortunately, it's one of the more visually distinctive spectra. But that can't really be...? Incredulous, I pulled up the newly identified element's spectrum and found, not just the two obvious lines, but no fewer than ten of its lines are visible in the photo. All of them line up pretty much exactly (diffraction gratings aren't perfect), and all are in exactly the right places relative to the nearest Sc, Hg, and Na lines.
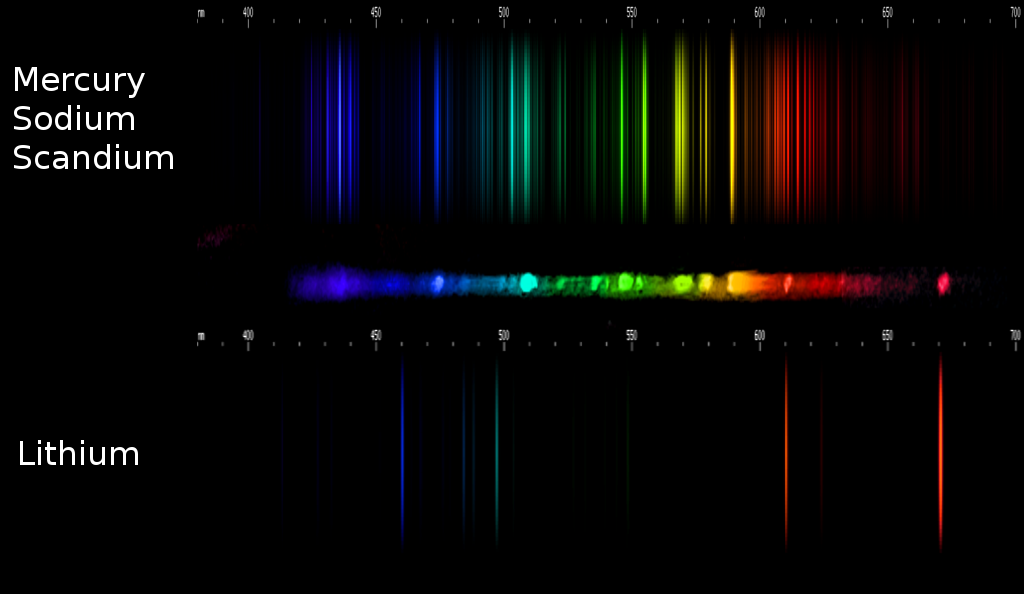
That's right, some MH lamps also contain lithium, and identifying it is easy enough: "look for two bright lines in the red region, near 671nm and 610nm."
So that is the importance of the "How to Identify" instructions for each element. They are useful not only when observing real samples, but also when playing the identification game, which I highly recommend for perfecting your spectrum identification skills.
 High Resolution Spectra |
Periodic Table of Spectra |
 Interactive Browser |
 Which Element Are You? |
 Screensaver Runs entirely in browser; No download, no risk. |